Metabolic pathways in immune cell activation and quiescence
- PMID: 23601682
- PMCID: PMC3654249
- DOI: 10.1016/j.immuni.2013.04.005
Metabolic pathways in immune cell activation and quiescence
Abstract
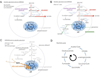
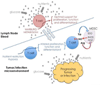
Studies of immune system metabolism ("immunometabolism") segregate along two paths. The first investigates the effects of immune cells on organs that regulate whole-body metabolism, such as adipose tissue and liver. The second explores the role of metabolic pathways within immune cells and how this regulates immune response outcome. Distinct metabolic pathways diverge and converge at many levels, and, therefore, cells face choices as to how to achieve their metabolic goals. There is interest in fully understanding how and why immune cells commit to particular metabolic fates and in elucidating the immunologic consequences of reaching a metabolic endpoint by one pathway versus another. This is particularly intriguing, given that metabolic commitment is influenced not only by substrate availability but also by signaling pathways elicited by metabolites. Thus, metabolic choices in cells enforce fate and function, and this area will be the subject of this review.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

