Comparison of clot-based, chromogenic and fluorescence assays for measurement of factor VIII inhibitors in the US Hemophilia Inhibitor Research Study
- PMID: 23601690
- PMCID: PMC4477744
- DOI: 10.1111/jth.12259
Comparison of clot-based, chromogenic and fluorescence assays for measurement of factor VIII inhibitors in the US Hemophilia Inhibitor Research Study
Abstract
Background: Detection and validation of inhibitors (antibodies) to hemophilia treatment products are important for clinical care, evaluation of product safety and assessment of population trends.
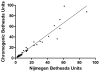
Methods: Centralized monitoring for factor VIII (FVIII) inhibitors was conducted for patients in the Hemophilia Inhibitor Research Study using a previously reported modified Nijmegen-Bethesda clotting assay (NBA), a chromogenic Bethesda assay (CBA) and a novel fluorescence immunoassay (FLI).
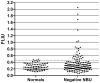
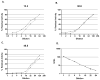
Results: NBA and CBA were performed on 1005 specimens and FLI on 272 specimens. CBA was negative on 880/883 specimens (99.7%) with Nijmegen-Bethesda units (NBU) < 0.5 and positive on 42/42 specimens (100%) with NBU ≥ 2.0 and 43/80 specimens (53.8%) with NBU 0.5-1.9. Among specimens with positive NBA and negative CBA, 58.1% were FLI negative, 12.9% had evidence of lupus anticoagulant, and 35.5% had non-time-dependent inhibition. CBA and FLI were positive on 72.4% and 100% of 1.0-1.9 NBU specimens and 43.1% and 50.0% of 0.5-0.9 NBU specimens. FLI detected antibodies in 98.0% of CBA-positive and 81.6% of NBA-positive specimens (P = 0.004). Among 21 new inhibitors detected by NBA, five (23.8%) with 0.7-1.3 NBU did not react in CBA or FLI. Among previously positive patients with 0.5-1.9 NBU, 7/25 (28%) were not CBA or FLI positive. FLI was positive on 36/169 NBU-negative specimens (21.3%).
Conclusions: FVIII specificity could not be demonstrated by CBA or FLI for 26% of inhibitors of 0.5-1.9 NBU; such results must be interpreted with caution. Low titer inhibitors detected in clot-based assays should always be repeated, with consideration given to evaluating their reactivity with FVIII using more specific assays.
Keywords: factor VIII; factor VIII inhibitor; hemophilia A; immunology and fluorescence immunoassay.
© 2013 International Society on Thrombosis and Haemostasis.
Figures


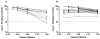
Similar articles
-
Limit of detection and threshold for positivity of the Centers for Disease Control and Prevention assay for factor VIII inhibitors.J Thromb Haemost. 2017 Oct;15(10):1971-1976. doi: 10.1111/jth.13795. Epub 2017 Sep 14. J Thromb Haemost. 2017. PMID: 28795528 Free PMC article.
-
Validation of Nijmegen-Bethesda assay modifications to allow inhibitor measurement during replacement therapy and facilitate inhibitor surveillance.J Thromb Haemost. 2012 Jun;10(6):1055-61. doi: 10.1111/j.1538-7836.2012.04705.x. J Thromb Haemost. 2012. PMID: 22435927 Free PMC article.
-
Characterization of the anti-factor VIII immunoglobulin profile in patients with hemophilia A by use of a fluorescence-based immunoassay.J Thromb Haemost. 2015 Jan;13(1):47-53. doi: 10.1111/jth.12768. Epub 2014 Dec 11. J Thromb Haemost. 2015. PMID: 25354263 Free PMC article.
-
A critical appraisal of one-stage and chromogenic assays of factor VIII activity.J Thromb Haemost. 2016 Feb;14(2):248-61. doi: 10.1111/jth.13215. Epub 2016 Feb 1. J Thromb Haemost. 2016. PMID: 26663865 Review.
-
Inhibitor antibodies to factor VIII and factor IX: management.Semin Thromb Hemost. 2000;26(2):179-88. doi: 10.1055/s-2000-9821. Semin Thromb Hemost. 2000. PMID: 10919411 Review.
Cited by
-
Investigating potential mechanisms underlying FVIII inhibition in acquired hemophilia A associated with mRNA COVID-19 vaccines.J Thromb Haemost. 2022 Apr;20(4):1015-1018. doi: 10.1111/jth.15665. Epub 2022 Feb 13. J Thromb Haemost. 2022. PMID: 35108443 Free PMC article. No abstract available.
-
Laboratory testing for factor VIII and IX inhibitors in haemophilia: A review.Haemophilia. 2018 Mar;24(2):186-197. doi: 10.1111/hae.13424. Epub 2018 Feb 15. Haemophilia. 2018. PMID: 29446525 Free PMC article. Review.
-
Emicizumab in tolerized patients with hemophilia A with inhibitors: A single-institution pediatric cohort assessing inhibitor status.Res Pract Thromb Haemost. 2021 Feb 8;5(2):342-348. doi: 10.1002/rth2.12475. eCollection 2021 Feb. Res Pract Thromb Haemost. 2021. PMID: 33733033 Free PMC article.
-
Clinical utility and impact of the use of the chromogenic vs one-stage factor activity assays in haemophilia A and B.Eur J Haematol. 2020 Jan;104(1):3-14. doi: 10.1111/ejh.13339. Epub 2019 Nov 13. Eur J Haematol. 2020. PMID: 31606899 Free PMC article. Review.
-
Improving the performance of factor VIII inhibitor tests in hemophilia A.Thromb Res. 2015 Dec;136(6):1047-8. doi: 10.1016/j.thromres.2015.09.019. Epub 2015 Sep 28. Thromb Res. 2015. PMID: 26432649 Free PMC article. No abstract available.
References
-
- Biggs R, Bidwell E. A method for the study of antihaemophilic globulin inhibitors with reference to six cases. Br J Haematol. 1959;5:379–95. - PubMed
-
- Nuss R, Jacobson L, Hathaway WE, Manco-Johnson M Recombinate PUP Study Group. Evidence for antiphospholipid antibodies in children with factor VIII inhibitors. Thromb Haemost. 1999;82:1559–60. - PubMed
-
- Blanco AN, Peirano AA, Grosso SH, Gennari LC, Perez Blanco R, Lazzari MA. A chromogenic substrate method for detecting and titrating anti-factor VIII antibodies in the presence of lupus anticoagulants. Haematologica. 2002;87:271–8. - PubMed
-
- Shaw PH, Reynolds S, Gunawardena S, Krishnamurti L, Ritchey AK. The prevalence of bleeding disorders among healthy pediatric patients with abnormal preprocedural coagulation studies. J Pediatr Hematol Oncol. 2008;30:135–41. - PubMed
-
- Manco-Johnson MJ, Nuss R, Jacobson LJ. Heparin neutralization is essential for accurate measurement of factor VIII activity and inhibitor assays in blood samples drawn from implanted venous access devices. J Lab Clin Med. 2000;136:74–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

