A comparative study of varying doses of enoxaparin for thromboprophylaxis in critically ill patients: a double-blinded, randomised controlled trial
- PMID: 23601744
- PMCID: PMC4057520
- DOI: 10.1186/cc12684
A comparative study of varying doses of enoxaparin for thromboprophylaxis in critically ill patients: a double-blinded, randomised controlled trial
Abstract
Introduction: Critically ill patients are predisposed to venous thromboembolism. We hypothesized that higher doses of enoxaparin would improve thromboprophylaxis without increasing the risk of bleeding. Peak anti-factor Xa (anti-Xa) levels of 0.1 to 0.4 IU/ml reflect adequate thromboprophylaxis for general ward patients. Studies conducted in orthopaedic patients demonstrated a statistically significant relationship between anti-Xa levels and wound haematoma and thrombosis. Corresponding levels for critically ill patients may well be higher, but have never been validated in large studies.
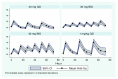
Methods: Eighty critically ill patients weighing 50 to 90 kilograms were randomised in a double-blinded study to receive subcutaneous (sc) enoxaparin: 40 mg once daily (QD), 30 mg twice daily (BID), 40 mg BID, or 1 mg/kg QD, each administered for three days. Anti-Xa activity was measured at baseline, and daily at 4, 12, 16 and 24 hours post administration. Antithrombin, fibrinogen, and platelets were measured at baseline and twice daily thereafter.
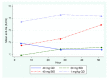
Results: Two patients were transferred prior to participation. On day 1, doses of 40 mg QD (n = 20) and 40 mg BID (n = 19) yielded mean peak anti-Xa of 0.20 IU/ml and 0.17 IU/ml respectively. A dose of 30 mg BID (n = 20) resulted in much lower levels (0.08 IU/ml). Patients receiving 1 mg/kg QD (n = 19) achieved near steady-state mean peak anti-Xa levels from day 1 (0.34 IU/ml). At steady state (day 3), mean peak anti-Xa levels of 0.13 IU/ml and 0.15 IU/ml were achieved with doses of 40 mg QD and 30 mg BID respectively. This increased significantly to 0.33 IU/ml and 0.40 IU/ml for doses of 40 mg BID and 1 mg/kg QD respectively. Thus anti-Xa response profiles differed significantly over the three days between enoxaparin treatment groups (P <0.0001). Doses of 40 mg BID and 1 mg/kg QD enoxaparin yielded target anti-Xa levels for over 80% of the study period. There were no adverse effects.
Conclusions: Doses of 40 mg QD enoxaparin (Europe) or 30 mg BID (North America) yield levels of anti-Xa which may be inadequate for critically ill patients. A weight-based dose yielded the best anti-Xa levels without bioaccumulation, and allowed the establishment of near steady-state levels from the first day of enoxaparin administration.
Trial registration: Current Controlled Trials ISRCTN91570009.
Figures
References
-
- Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;17:381S–453S. doi: 10.1378/chest.08-0656. - DOI - PubMed
-
- Shorr AF, Williams MD. Venous thromboembolism in critically ill patients. Observations from a randomized trial in sepsis. Thromb Haemost. 2009;17:139–144. - PubMed
-
- Cook DJ, Crowther MA. Thromboprophylaxis in the intensive care unit: focus on medical-surgical patients. Crit Care Med. 2010;17:S76–S82. - PubMed
-
- PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group; Cook D, Meade M, Guyatt G, Walter S, Heels-Ansdell D, Warkentin TE, Zytaruk N, Crowther M, Geerts W, Cooper DJ, Vallance S, Qushmaq I, Rocha M, Berwanger O, Vlahakis NE. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;17:1305–1314. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical