Risk of elevated intraocular pressure and glaucoma in patients with uveitis: results of the multicenter uveitis steroid treatment trial
- PMID: 23601801
- PMCID: PMC3720698
- DOI: 10.1016/j.ophtha.2013.01.025
Risk of elevated intraocular pressure and glaucoma in patients with uveitis: results of the multicenter uveitis steroid treatment trial
Abstract
Objective: To report the 2-year incidence of raised intraocular pressure (IOP) and glaucomatous optic nerve damage in patients with uveitis randomized to either fluocinolone acetonide (FA) implants or systemic therapy. Secondarily, we sought to explore patient and eye characteristics associated with IOP elevation or nerve damage.
Design: A randomized, partially masked trial in which patients were randomized to either FA implants or systemic therapy.
Participants: Patients aged ≥ 13 years with noninfectious intermediate, posterior, or panuveitis active within the prior 60 days for which systemic corticosteroids were indicated were eligible.
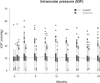
Methods: Visual fields were obtained at baseline and every 12 months using the Humphrey 24-2 Swedish interactive threshold algorithm (SITA) fast protocol. Stereoscopic optic nerve photos were taken at baseline and at 3-, 6-, 12-, and 24-month follow-up visits. Masked examiners measured IOP at every study visit.
Main outcome measures: Glaucoma was diagnosed based on an increase in optic nerve cup-to-disc ratio with visual field worsening or increased cup-to-disc ratio alone, for cases where visual field change was not evaluable, because of missing data or severe visual field loss at baseline.
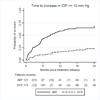
Results: Most patients were treated as assigned; among those evaluated for glaucoma, 97% and 10% of patients assigned to implant and systemic treatment, respectively, received implants. More patients (65%) assigned to implants experienced an IOP elevation of ≥ 10 mmHg versus 24% assigned to systemic treatment (P<0.001). Similarly, 69% of patients assigned to the implant required IOP-lowering therapy versus 26% in the systemic group (P<0.001). Glaucomatous optic nerve damage developed in 23% versus 6% (P<0.001) of implant and systemic patients, respectively. In addition to treatment assignment, black race, use of IOP-lowering medications, and uveitis activity at baseline were associated with incident glaucoma (P<0.05).
Conclusions: Implant-assigned eyes had about a 4-fold risk of developing IOP elevation of ≥ 10 mmHg and incident glaucomatous optic neuropathy over the first 2 years compared with those assigned to systemic therapy. Central visual acuity was unaffected. Aggressive IOP monitoring with early treatment (often including early filtration surgery) is needed to avoid glaucoma when vision-threatening inflammation requires implant therapy.
Financial disclosure(s): Proprietary or commercial disclosure may be found after the references.
Copyright © 2013 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Goldstein DA, Godfrey DG, Hall A, et al. Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Arch Ophthalmol. 2007;125:1478–1485. - PubMed
-
- Leder HA, Jabs DA, Galor A, et al. Periocular triamcinolone acetonide injections for cystoid macular edema complicating noninfectious uveitis. Am J Ophthalmol. 2011;152:441–448. - PubMed
-
- Ferrante P, Ramsey A, Bunce C, Lightman S. Clinical trial to compare efficacy and side-effects of injection of posterior sub-Tenon triamcinolone versus orbital floor methylprednisolone in the management of posterior uveitis. Clin Experiment Ophthalmol. 2004;32:563–568. - PubMed
-
- Lowder C, Belfort R, Jr, Lightman S, et al. Ozurdex HURON Study Group. Dexamethasone intravitreal implant in the treatment of noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545–553. - PubMed
-
- Sallam A, Sheth HG, Habot-Wilner Z, Lightman S. Outcome of raised intraocular pressure in uveitic eyes with and without a corticosteroid-induced hypertensive response. Am J Ophthalmol. 2009;148:207–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

