The iron link between malaria and invasive non-typhoid Salmonella infections
- PMID: 23601932
- PMCID: PMC4521076
- DOI: 10.1016/j.pt.2013.03.006
The iron link between malaria and invasive non-typhoid Salmonella infections
Abstract
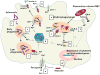
Epidemiological studies have demonstrated an association between malaria and invasive non-typhoid Salmonella (NTS) infections, especially in children. We explore the role of iron as a possible cofactor in this association. Malarial disease, among others, is associated with enhanced erythrophagocytosis and inflammation, which increases the iron content of macrophages and thereby also the survival of Salmonella spp. within macrophages. Whether iron supplementation programs augment the risk of invasive NTS infections in malaria-endemic regions is an important global health issue that still needs to be determined.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Malaria parasite infection compromises control of concurrent systemic non-typhoidal Salmonella infection via IL-10-mediated alteration of myeloid cell function.PLoS Pathog. 2014 May 1;10(5):e1004049. doi: 10.1371/journal.ppat.1004049. eCollection 2014 May. PLoS Pathog. 2014. PMID: 24787713 Free PMC article.
-
Immunological bases of increased susceptibility to invasive nontyphoidal Salmonella infection in children with malaria and anaemia.Microbes Infect. 2018 Oct-Nov;20(9-10):589-598. doi: 10.1016/j.micinf.2017.11.014. Epub 2017 Dec 15. Microbes Infect. 2018. PMID: 29248635 Free PMC article. Review.
-
Loss of Humoral and Cellular Immunity to Invasive Nontyphoidal Salmonella during Current or Convalescent Plasmodium falciparum Infection in Malawian Children.Clin Vaccine Immunol. 2017 Jul 5;24(7):e00057-17. doi: 10.1128/CVI.00057-17. Print 2017 Jul. Clin Vaccine Immunol. 2017. PMID: 28515136 Free PMC article.
-
Infectious disease: Opposing effects of IL-10.Nat Rev Immunol. 2014 Jun;14(6):356. doi: 10.1038/nri3693. Nat Rev Immunol. 2014. PMID: 24854586 No abstract available.
-
The association between malaria and non-typhoid Salmonella bacteraemia in children in sub-Saharan Africa: a literature review.Malar J. 2014 Oct 13;13:400. doi: 10.1186/1475-2875-13-400. Malar J. 2014. PMID: 25311375 Free PMC article. Review.
Cited by
-
Epidemic increase in Salmonella bloodstream infection in children, Bwamanda, the Democratic Republic of Congo.Eur J Clin Microbiol Infect Dis. 2014 Jan;33(1):79-87. doi: 10.1007/s10096-013-1931-8. Epub 2013 Aug 24. Eur J Clin Microbiol Infect Dis. 2014. PMID: 23975545
-
Role of Activins in Hepcidin Regulation during Malaria.Infect Immun. 2017 Nov 17;85(12):e00191-17. doi: 10.1128/IAI.00191-17. Print 2017 Dec. Infect Immun. 2017. PMID: 28893916 Free PMC article.
-
Characterization of the Invasive, Multidrug Resistant Non-typhoidal Salmonella Strain D23580 in a Murine Model of Infection.PLoS Negl Trop Dis. 2015 Jun 19;9(6):e0003839. doi: 10.1371/journal.pntd.0003839. eCollection 2015 Jun. PLoS Negl Trop Dis. 2015. PMID: 26091096 Free PMC article.
-
Dissemination of non-typhoidal Salmonella during Plasmodium chabaudi infection affects anti-malarial immunity.Parasitol Res. 2019 Jul;118(7):2277-2285. doi: 10.1007/s00436-019-06349-z. Epub 2019 May 23. Parasitol Res. 2019. PMID: 31119381 Free PMC article.
-
Disrupted Iron Metabolism and Mortality during Co-infection with Malaria and an Intestinal Gram-Negative Extracellular Pathogen.Cell Rep. 2021 Jan 12;34(2):108613. doi: 10.1016/j.celrep.2020.108613. Cell Rep. 2021. PMID: 33440153 Free PMC article.
References
-
- McLean E, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12:444–454. - PubMed
-
- Sazawal S, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

