Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: implications for age-related degenerative diseases of the eye
- PMID: 23601964
- PMCID: PMC3947380
- DOI: 10.1016/j.cyto.2013.03.027
Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: implications for age-related degenerative diseases of the eye
Abstract
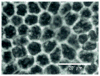
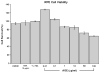
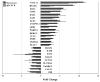
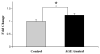
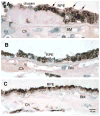
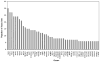
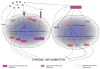
Age related macular degeneration (AMD) is one of the leading causes of blindness in Western society. A hallmark of early stage AMD are drusen, extracellular deposits that accumulate in the outer retina. Advanced glycation endproducts (AGE) accumulate with aging and are linked to several age-related diseases such as Alzheimer's disease, osteoarthritis, atherosclerosis and AMD. AGE deposits are found in drusen and in Bruch's membrane of the eye and several studies have suggested its role in promoting oxidative stress, apoptosis and lipofuscin accumulation. Recently, complement activation and chronic inflammation have been implicated in the pathogenesis of AMD. While AGEs have been shown to promote inflammation in other diseases, whether it plays a similar role in AMD is not known. This study investigates the effects of AGE stimulation on pro- and anti-inflammatory pathways in primary culture of human retinal pigment epithelial cells (RPE). Differential gene expression studies revealed a total of 41 up- and 18 down-regulated RPE genes in response to AGE stimulation. These genes fell into three categories as assessed by gene set enrichment analysis (GSEA). The main categories were inflammation (interferon-induced, immune response) and proteasome degradation, followed by caspase signaling. Using suspension array technology, protein levels of secreted cytokines and growth factors were also examined. Anti-inflammatory cytokines including IL10, IL1ra and IL9 were all overexpressed. Pro-inflammatory cytokines including IL4, IL15 and IFN-γ were overexpressed, while other pro-inflammatory cytokines including IL8, MCP1, IP10 were underexpressed after AGE stimulation, suggesting a para-inflammation state of the RPE under these conditions. Levels of mRNA of chemokine, CXCL11, and viperin, RSAD2, were up-regulated and may play a role in driving the inflammatory response via the NF-kB and JAK-STAT pathways. CXCL11 was strongly immunoreactive and associated with drusen in the AMD eye. The pathways and novel genes identified here highlight inflammation as a key response to AGE stimulation in primary culture of human RPE, and identify chemokine CXCL11 as putative novel agent associated with the pathogenesis of AMD.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. - PubMed
-
- Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

