HIV-1 reverse transcriptase and antiviral drug resistance. Part 1
- PMID: 23602471
- PMCID: PMC4097814
- DOI: 10.1016/j.coviro.2013.03.012
HIV-1 reverse transcriptase and antiviral drug resistance. Part 1
Abstract
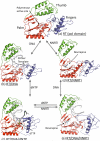
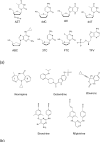
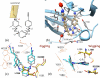
HIV-1 reverse transcriptase (RT) contributes to the development of resistance to all anti-AIDS drugs by introducing mutations into the viral genome. At the molecular level, mutations in RT result in resistance to RT inhibitors. Eight nucleoside/nucleotide analogs (NRTIs) and five non-nucleoside inhibitors (NNRTIs) are approved HIV-1 drugs. Structures of RT have been determined in complexes with substrates and/or inhibitors, and the structures have illuminated different conformational and functional states of the enzyme. Understanding the molecular mechanisms of resistance to NRTIs and NNRTIs, and their complex relationships, may help in designing new drugs that are periodically required to overcome existing as well as emerging trends of drug resistance.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures
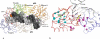
References
-
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983;220:868–871. - PubMed
-
- Gallo RC, Salahuddin SZ, Popovic M, Shearer GM, Kaplan M, Haynes BF, Palker TJ, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. - PubMed
-
- Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrman SN, Gallo RC, Bolognesi D, Barry DW, Broder S. 3′-Azido-3′-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985;82:7096–7100. - PMC - PubMed
-
- Fischl MA, Richman DD, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, Groopman JE, Mildvan D, Schooley RT, et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317:185–191. - PubMed
-
- Grobler JA, Stillmock K, Hu B, Witmer M, Felock P, Espeseth AS, Wolfe A, Egbertson M, Bourgeois M, Melamed J, et al. Diketo acid inhibitor mechanism and HIV-1 integrase: implications for metal binding in the active site of phosphotransferase enzymes. Proc Natl Acad Sci U S A. 2002;99:6661–6666. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

