Arsenite-induced ROS/RNS generation causes zinc loss and inhibits the activity of poly(ADP-ribose) polymerase-1
- PMID: 23602911
- PMCID: PMC3766412
- DOI: 10.1016/j.freeradbiomed.2013.04.019
Arsenite-induced ROS/RNS generation causes zinc loss and inhibits the activity of poly(ADP-ribose) polymerase-1
Abstract
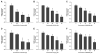
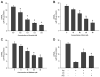
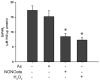
Arsenic enhances the genotoxicity of other carcinogenic agents such as ultraviolet radiation and benzo[a]pyrene. Recent reports suggest that inhibition of DNA repair is an important aspect of arsenic cocarcinogenesis, and DNA repair proteins such as poly(ADP ribose) polymerase (PARP)-1 are direct molecular targets of arsenic. Although arsenic has been shown to generate reactive oxygen/nitrogen species (ROS/RNS), little is known about the role of arsenic-induced ROS/RNS in the mechanism underlying arsenic inhibition of DNA repair. We report herein that arsenite-generated ROS/RNS inhibits PARP-1 activity in cells. Cellular exposure to arsenite, as well as hydrogen peroxide and NONOate (nitric oxide donor), decreased PARP-1 zinc content, enzymatic activity, and PARP-1 DNA binding. Furthermore, the effects of arsenite on PARP-1 activity, DNA binding, and zinc content were partially reversed by the antioxidant ascorbic acid, catalase, and the NOS inhibitor, aminoguanidine. Most importantly, arsenite incubation with purified PARP-1 protein in vitro did not alter PARP-1 activity or DNA-binding ability, whereas hydrogen peroxide or NONOate retained PARP-1 inhibitory activity. These results strongly suggest that cellular generation of ROS/RNS plays an important role in arsenite inhibition of PARP-1 activity, leading to the loss of PARP-1 DNA-binding ability and enzymatic activity.
Keywords: Arsenic; PARP-1; RNS; ROS.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

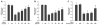



Similar articles
-
S-nitrosation on zinc finger motif of PARP-1 as a mechanism of DNA repair inhibition by arsenite.Oncotarget. 2016 Dec 6;7(49):80482-80492. doi: 10.18632/oncotarget.12613. Oncotarget. 2016. PMID: 27741521 Free PMC article.
-
Peroxynitrite contributes to arsenic-induced PARP-1 inhibition through ROS/RNS generation.Toxicol Appl Pharmacol. 2019 Sep 1;378:114602. doi: 10.1016/j.taap.2019.114602. Epub 2019 May 29. Toxicol Appl Pharmacol. 2019. PMID: 31152818 Free PMC article.
-
Dual actions involved in arsenite-induced oxidative DNA damage.Chem Res Toxicol. 2008 Sep;21(9):1806-13. doi: 10.1021/tx8001548. Epub 2008 Aug 16. Chem Res Toxicol. 2008. PMID: 18707137 Free PMC article.
-
Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders.Cardiovasc Toxicol. 2018 Dec;18(6):493-506. doi: 10.1007/s12012-018-9462-2. Cardiovasc Toxicol. 2018. PMID: 29968072 Review.
-
Neuronal trauma model: in search of Thanatos.Int J Dev Neurosci. 2004 Nov;22(7):485-96. doi: 10.1016/j.ijdevneu.2004.07.015. Int J Dev Neurosci. 2004. PMID: 15465278 Review.
Cited by
-
Arsenic co-carcinogenesis: Inhibition of DNA repair and interaction with zinc finger proteins.Semin Cancer Biol. 2021 Nov;76:86-98. doi: 10.1016/j.semcancer.2021.05.009. Epub 2021 May 10. Semin Cancer Biol. 2021. PMID: 33984503 Free PMC article. Review.
-
Oxidative Injury in Ischemic Stroke: A Focus on NADPH Oxidase 4.Oxid Med Cell Longev. 2022 Feb 3;2022:1148874. doi: 10.1155/2022/1148874. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35154560 Free PMC article. Review.
-
Roles of UVA radiation and DNA damage responses in melanoma pathogenesis.Environ Mol Mutagen. 2018 Jun;59(5):438-460. doi: 10.1002/em.22176. Epub 2018 Feb 21. Environ Mol Mutagen. 2018. PMID: 29466611 Free PMC article. Review.
-
S-nitrosation on zinc finger motif of PARP-1 as a mechanism of DNA repair inhibition by arsenite.Oncotarget. 2016 Dec 6;7(49):80482-80492. doi: 10.18632/oncotarget.12613. Oncotarget. 2016. PMID: 27741521 Free PMC article.
-
Coordinate H3K9 and DNA methylation silencing of ZNFs in toxicant-induced malignant transformation.Epigenetics. 2013 Oct;8(10):1080-8. doi: 10.4161/epi.25926. Epub 2013 Aug 6. Epigenetics. 2013. PMID: 23974009 Free PMC article.
References
-
- Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nature reviews. Molecular cell biology. 2006;7:517–528. - PubMed
-
- Christmann M, Tomicic MT, Roos WP, Kaina B. Mechanisms of human DNA repair: an update. Toxicology. 2003;193:3–34. - PubMed
-
- Burkle A, Diefenbach J, Brabeck C, Beneke S. Ageing and PARP. Pharmacological research: the official journal of the Italian Pharmacological Society. 2005;52:93–99. - PubMed
-
- Virag L. Structure and function of poly(ADP-ribose) polymerase-1: role in oxidative stress-related pathologies. Current vascular pharmacology. 2005;3:209–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

