Adaptation maintains population homeostasis in primary visual cortex
- PMID: 23603708
- PMCID: PMC3665725
- DOI: 10.1038/nn.3382
Adaptation maintains population homeostasis in primary visual cortex
Abstract
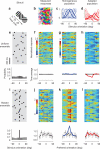
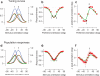
Sensory systems exhibit mechanisms of neural adaptation, which adjust neuronal activity on the basis of recent stimulus history. In primary visual cortex (V1) in particular, adaptation controls the responsiveness of individual neurons and shifts their visual selectivity. What benefits does adaptation confer on a neuronal population? We measured adaptation in the responses of populations of cat V1 neurons to stimulus ensembles with markedly different statistics of stimulus orientation. We found that adaptation served two homeostatic goals. First, it maintained equality in the time-averaged responses across the population. Second, it maintained independence in selectivity across the population. Adaptation scaled and distorted population activity according to a simple multiplicative rule that depended on neuronal orientation preference and on stimulus orientation. We conclude that adaptation in V1 acts as a mechanism of homeostasis, enforcing a tendency toward equality and independence in neural activity across the population.
Figures



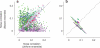
Comment in
-
Equalization and decorrelation in primary visual cortex.J Neurophysiol. 2014 Aug 1;112(3):501-3. doi: 10.1152/jn.00521.2013. Epub 2014 Feb 26. J Neurophysiol. 2014. PMID: 24572090
References
-
- Anstis S, Verstraten FA, Mather G. The motion aftereffect. Trends in cognitive sciences. 1998;2:111–117. - PubMed
-
- Gibson JJ, Radner M. Adaptation, after-effect, and contrast in the perception of tilted lines: I. Quantitative studies. Journal of Experimental Psychology. 1937;20:453–467.
-
- Jin DZ, Dragoi V, Sur M, Seung HS. Tilt aftereffect and adaptation-induced changes in orientation tuning in visual cortex. J Neurophysiol. 2005;94:4038–4050. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

