Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification
- PMID: 23603848
- PMCID: PMC5833942
- DOI: 10.1038/nmat3627
Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification
Abstract
The accumulation of calcified material in cardiovascular tissue is thought to involve cytochemical, extracellular matrix and systemic signals; however, its precise composition and nanoscale architecture remain largely unexplored. Using nano-analytical electron microscopy techniques, we examined valves, aortae and coronary arteries from patients with and without calcific cardiovascular disease and detected spherical calcium phosphate particles, regardless of the presence of calcific lesions. We also examined lesions after sectioning with a focused ion beam and found that the spherical particles are composed of highly crystalline hydroxyapatite that crystallographically and structurally differs from bone mineral. Taken together, these data suggest that mineralized spherical particles may play a fundamental role in calcific lesion formation. Their ubiquitous presence in varied cardiovascular tissues and from patients with a spectrum of diseases further suggests that lesion formation may follow a common process. Indeed, applying materials science techniques to ectopic and orthotopic calcification has great potential to lend critical insights into pathophysiological processes underlying calcific cardiovascular disease.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
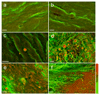


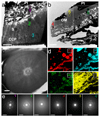
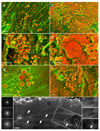
Comment in
-
Cardiovascular calcification: Orbicular origins.Nat Mater. 2013 Jun;12(6):476-8. doi: 10.1038/nmat3663. Nat Mater. 2013. PMID: 23695741 No abstract available.
References
-
- Butler D. UN targets top killers. Nature. 2011:260–261. - PubMed
-
- Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. - PubMed
-
- Nkomo VT, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

