Endoplasmic reticulum stress regulates rat mandibular cartilage thinning under compressive mechanical stress
- PMID: 23603905
- PMCID: PMC3689960
- DOI: 10.1074/jbc.M112.407296
Endoplasmic reticulum stress regulates rat mandibular cartilage thinning under compressive mechanical stress
Abstract
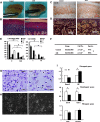
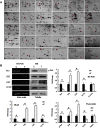
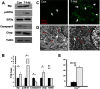
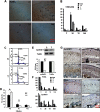
Compressive mechanical stress-induced cartilage thinning has been characterized as a key step in the progression of temporomandibular joint diseases, such as osteoarthritis. However, the regulatory mechanisms underlying this loss have not been thoroughly studied. Here, we used an established animal model for loading compressive mechanical stress to induce cartilage thinning in vivo. The mechanically stressed mandibular chondrocytes were then isolated to screen potential candidates using a proteomics approach. A total of 28 proteins were identified that were directly or indirectly associated with endoplasmic reticulum stress, including protein disulfide-isomerase, calreticulin, translationally controlled tumor protein, and peptidyl-prolyl cis/trans-isomerase protein. The altered expression of these candidates was validated at both the mRNA and protein levels. The induction of endoplasmic reticulum stress by mechanical stress loading was confirmed by the activation of endoplasmic reticulum stress markers, the elevation of the cytoplasmic Ca(2+) level, and the expansion of endoplasmic reticulum membranes. More importantly, the use of a selective inhibitor to block endoplasmic reticulum stress in vivo reduced the apoptosis observed at the early stages of mechanical stress loading and inhibited the proliferation observed at the later stages of mechanical stress loading. Accordingly, the use of the inhibitor significantly restored cartilage thinning. Taken together, these results demonstrated that endoplasmic reticulum stress is significantly activated in mechanical stress-induced mandibular cartilage thinning and, more importantly, that endoplasmic reticulum stress inhibition alleviates this loss, suggesting a novel pharmaceutical strategy for the treatment of mechanical stress-induced temporomandibular joint diseases.
Keywords: Cartilage; Chondrocytes; ER Stress; Mechanotransduction; Proteomics.
Figures



References
-
- Sicher H. (1947) The growth of the mandible. Am. J. Orthod. 43, 123–128 - PubMed
-
- Kiliaridis S., Thilander B., Kjellberg H., Topouzelis N., Zafiriadis A. (1999) Effect of low masticatory function on condylar growth. A morphometric study in the rat. Am. J. Orthod. Dentofacial Orthop. 116, 121–125 - PubMed
-
- Murray R. C., Zhu C. F., Goodship A. E., Lakhani K. H., Agrawal C. M., Athanasiou K. A. (1999) Exercise affects the mechanical properties and histological appearance of equine articular cartilage. J. Orthop. Res. 17, 725–731 - PubMed
-
- Kuroda S., Tanimoto K., Izawa T., Fujihara S., Koolstra J. H., Tanaka E. (2009) Biomechanical and biochemical characteristics of the mandibular condylar cartilage. Osteoarthr. Cartil. 17, 1408–1415 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

