The choline-binding protein PspC of Streptococcus pneumoniae interacts with the C-terminal heparin-binding domain of vitronectin
- PMID: 23603906
- PMCID: PMC3668722
- DOI: 10.1074/jbc.M112.443507
The choline-binding protein PspC of Streptococcus pneumoniae interacts with the C-terminal heparin-binding domain of vitronectin
Abstract
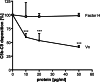
Adherence of Streptococcus pneumoniae is directly mediated by interactions of adhesins with eukaryotic cellular receptors or indirectly by exploiting matrix and serum proteins as molecular bridges. Pneumococci engage vitronectin, the human adhesive glycoprotein and complement inhibitor, to facilitate attachment to epithelial cells of the mucosal cavity, thereby modulating host cell signaling. In this study, we identified PspC as a vitronectin-binding protein interacting with the C-terminal heparin-binding domain of vitronectin. PspC is a multifunctional surface-exposed choline-binding protein displaying various adhesive properties. Vitronectin binding required the R domains in the mature PspC protein, which are also essential for the interaction with the ectodomain of the polymeric immunoglobulin receptor and secretory IgA. Consequently, secretory IgA competitively inhibited binding of vitronectin to purified PspC and to PspC-expressing pneumococci. In contrast, Factor H, which binds to the N-terminal part of mature PspC molecules, did not interfere with the PspC-vitronectin interaction. Using a series of vitronectin peptides, the C-terminal heparin-binding domain was shown to be essential for the interaction of soluble vitronectin with PspC. Binding experiments with immobilized vitronectin suggested a region N-terminal to the identified heparin-binding domain as an additional binding region for PspC, suggesting that soluble, immobilized, as well as cellularly bound vitronectin possesses different conformations. Finally, vitronectin bound to PspC was functionally active and inhibited the deposition of the terminal complement complex. In conclusion, this study identifies and characterizes (on the molecular level) the interaction between the pneumococcal adhesin PspC and the human glycoprotein vitronectin.
Keywords: Adhesin; Bacterial Pathogenesis; Complement; Extracellular Matrix; Pneumococci; Protein-Protein Interactions; PspC; Surface Plasmon Resonance (SPR); Virulence Factors.
Figures


Similar articles
-
Binding of vitronectin and Factor H to Hic contributes to immune evasion of Streptococcus pneumoniae serotype 3.Thromb Haemost. 2015 Jan;113(1):125-42. doi: 10.1160/TH14-06-0561. Epub 2014 Aug 28. Thromb Haemost. 2015. PMID: 25181963
-
Serotype 3 pneumococci sequester platelet-derived human thrombospondin-1 via the adhesin and immune evasion protein Hic.J Biol Chem. 2017 Apr 7;292(14):5770-5783. doi: 10.1074/jbc.M116.760504. Epub 2017 Feb 16. J Biol Chem. 2017. PMID: 28209711 Free PMC article.
-
Streptococcus pneumoniae Binds to Host Lactate Dehydrogenase via PspA and PspC To Enhance Virulence.mBio. 2021 May 4;12(3):e00673-21. doi: 10.1128/mBio.00673-21. mBio. 2021. PMID: 33947761 Free PMC article.
-
Heterologous expression of pneumococcal virulence factor PspC on the surface of Lactococcus lactis confers adhesive properties.Microbiology (Reading). 2012 Mar;158(Pt 3):771-780. doi: 10.1099/mic.0.053603-0. Epub 2012 Jan 5. Microbiology (Reading). 2012. PMID: 22222496
-
Pneumococcal Adhesins PavB and PspC Are Important for the Interplay with Human Thrombospondin-1.J Biol Chem. 2015 Jun 5;290(23):14542-55. doi: 10.1074/jbc.M114.623876. Epub 2015 Apr 20. J Biol Chem. 2015. PMID: 25897078 Free PMC article.
Cited by
-
Role of Streptococcus pneumoniae Proteins in Evasion of Complement-Mediated Immunity.Front Microbiol. 2017 Feb 20;8:224. doi: 10.3389/fmicb.2017.00224. eCollection 2017. Front Microbiol. 2017. PMID: 28265264 Free PMC article. Review.
-
Microbial evasion of the complement system: a continuous and evolving story.Front Immunol. 2024 Jan 4;14:1281096. doi: 10.3389/fimmu.2023.1281096. eCollection 2023. Front Immunol. 2024. PMID: 38239357 Free PMC article. Review.
-
Hijacking Complement Regulatory Proteins for Bacterial Immune Evasion.Front Microbiol. 2016 Dec 20;7:2004. doi: 10.3389/fmicb.2016.02004. eCollection 2016. Front Microbiol. 2016. PMID: 28066340 Free PMC article. Review.
-
Treatment of Pneumococcal Infection by Using Engineered Human C-Reactive Protein in a Mouse Model.Front Immunol. 2020 Oct 7;11:586669. doi: 10.3389/fimmu.2020.586669. eCollection 2020. Front Immunol. 2020. PMID: 33117400 Free PMC article.
-
Impact of the glpQ2 gene on virulence in a Streptococcus pneumoniae serotype 19A sequence type 320 strain.Infect Immun. 2015 Feb;83(2):682-92. doi: 10.1128/IAI.02357-14. Epub 2014 Nov 24. Infect Immun. 2015. PMID: 25422269 Free PMC article.
References
-
- Cartwright K. (2002) Pneumococcal disease in Western Europe: burden of disease, antibiotic resistance, and management. Eur. J. Pediatr. 161, 188–195 - PubMed
-
- Anderton J. M., Rajam G., Romero-Steiner S., Summer S., Kowalczyk A. P., Carlone G. M., Sampson J. S., Ades E. W. (2007) E-cadherin is a receptor for the common protein pneumococcal surface adhesin A (PsaA) of Streptococcus pneumoniae. Microb. Pathog. 42, 225–236 - PubMed
-
- Bergmann S., Lang A., Rohde M., Agarwal V., Rennemeier C., Grashoff C., Preissner K. T., Hammerschmidt S. (2009) Integrin-linked kinase is required for vitronectin-mediated internalization of Streptococcus pneumoniae by host cells. J. Cell Sci. 122, 256–267 - PubMed
-
- Romero-Steiner S., Caba J., Rajam G., Langley T., Floyd A., Johnson S. E., Sampson J. S., Carlone G. M., Ades E. (2006) Adherence of recombinant pneumococcal surface adhesin A (rPsaA)-coated particles to human nasopharyngeal epithelial cells for the evaluation of anti-PsaA functional antibodies. Vaccine 24, 3224–3231 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

