NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro
- PMID: 23603977
- PMCID: PMC4002877
- DOI: 10.1038/aps.2013.22
NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro
Abstract
Aim: NVP-BEZ235 is a novel dual PI3K/mTOR inhibitor and shows dramatic effects on gliomas. The aim of this study was to investigate the effects of NVP-BEZ235 on the radiosensitivity and autophagy of glioma stem cells (GSCs) in vitro.
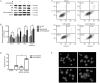
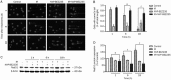
Methods: Human GSCs (SU-2) were tested. The cell viability and survival from ionizing radiation (IR) were evaluated using MTT and clonogenic survival assay, respectively. Immunofluorescence assays were used to identify the formation of autophagosomes. The apoptotic cells were quantified with annexin V-FITC/PI staining and flow cytometry, and observed using Hoechst 33258 staining and fluorescence microscope. Western blot analysis was used to analyze the expression levels of proteins. Cell cycle status was determined by measuring DNA content after staining with PI. DNA repair in the cells was assessed using a comet assay.
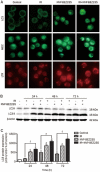
Results: Treatment of SU-2 cells with NVP-BEZ235 (10-320 nmol/L) alone suppressed the cell growth in a concentration-dependent manner. A low concentration of NVP-BEZ235 (10 nmol/L) significantly increased the radiation sensitivity of SU-2 cells, which could be blocked by co-treatment with 3-MA (50 μmol/L). In NVP-BEZ235-treated SU-2 cells, more punctate patterns of microtubule-associated protein LC3 immunoreactivity was observed, and the level of membrane-bound LC3-II was significantly increased. A combination of IR with NVP-BEZ235 significantly increased the apoptosis of SU-2 cells, as shown in the increased levels of BID, Bax, and active caspase-3, and decreased level of Bcl-2. Furthermore, the combination of IR with NVP-BEZ235 led to G1 cell cycle arrest. Moreover, NVP-BEZ235 significantly attenuated the repair of IR-induced DNA damage as reflected by the tail length of the comet.
Conclusion: NVP-BEZ235 increases the radiosensitivity of GSCs in vitro by activating autophagy that is associated with synergistic increase of apoptosis and cell-cycle arrest and decrease of DNA repair capacity.
Figures


Similar articles
-
NVP-BEZ235, a novel dual PI3K-mTOR inhibitor displays anti-glioma activity and reduces chemoresistance to temozolomide in human glioma cells.Cancer Lett. 2015 Oct 10;367(1):58-68. doi: 10.1016/j.canlet.2015.07.007. Epub 2015 Jul 15. Cancer Lett. 2015. PMID: 26188279
-
Simultaneous perturbation of the MAPK and the PI3K/mTOR pathways does not lead to increased radiosensitization.Radiat Oncol. 2015 Oct 24;10:214. doi: 10.1186/s13014-015-0514-5. Radiat Oncol. 2015. PMID: 26498922 Free PMC article.
-
Chloroquine, an autophagy inhibitor, potentiates the radiosensitivity of glioma initiating cells by inhibiting autophagy and activating apoptosis.BMC Neurol. 2016 Sep 20;16(1):178. doi: 10.1186/s12883-016-0700-6. BMC Neurol. 2016. PMID: 27644442 Free PMC article.
-
Targeting the RTK-PI3K-mTOR axis in malignant glioma: overcoming resistance.Curr Top Microbiol Immunol. 2010;347:279-96. doi: 10.1007/82_2010_67. Curr Top Microbiol Immunol. 2010. PMID: 20535652 Free PMC article. Review.
-
Targeting tumor hypoxia and mitochondrial metabolism with anti-parasitic drugs to improve radiation response in high-grade gliomas.J Exp Clin Cancer Res. 2020 Oct 7;39(1):208. doi: 10.1186/s13046-020-01724-6. J Exp Clin Cancer Res. 2020. PMID: 33028364 Free PMC article. Review.
Cited by
-
ATM, ATR, CHK1, CHK2 and WEE1 inhibitors in cancer and cancer stem cells.Medchemcomm. 2016 Nov 30;8(2):295-319. doi: 10.1039/c6md00439c. eCollection 2017 Feb 1. Medchemcomm. 2016. PMID: 30108746 Free PMC article. Review.
-
Radiation oncology in vitro: trends to improve radiotherapy through molecular targets.Biomed Res Int. 2014;2014:461687. doi: 10.1155/2014/461687. Epub 2014 Sep 15. Biomed Res Int. 2014. PMID: 25302298 Free PMC article. Review.
-
Introducing HDAC-Targeting Radiopharmaceuticals for Glioblastoma Imaging and Therapy.Pharmaceuticals (Basel). 2023 Feb 1;16(2):227. doi: 10.3390/ph16020227. Pharmaceuticals (Basel). 2023. PMID: 37259375 Free PMC article. Review.
-
Brain tumor stem cells: Molecular characteristics and their impact on therapy.Mol Aspects Med. 2014 Oct;39:82-101. doi: 10.1016/j.mam.2013.06.004. Epub 2013 Jul 4. Mol Aspects Med. 2014. PMID: 23831316 Free PMC article. Review.
-
The resveratrol analogue, HS‑1793, enhances the effects of radiation therapy through the induction of anti‑tumor immunity in mammary tumor growth.Int J Oncol. 2020 Jun;56(6):1405-1416. doi: 10.3892/ijo.2020.5017. Epub 2020 Mar 19. Int J Oncol. 2020. PMID: 32236622 Free PMC article.
References
-
- Tunici P, Irvin D, Liu G, Yuan X, Zhaohui Z, Ng H, et al. Brain tumor stem cells: new targets for clinical treatments. Neurosurg Focus. 2006;20:E27. - PubMed
-
- Gupta T, Nair V, Paul SN, Kannan S, Moiyadi A, Epari S, et al. Can irradiation of potential cancer stem-cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma. J Neurooncol. 2012;109:195–203. - PubMed
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

