The nascent polypeptide-associated complex is a key regulator of proteostasis
- PMID: 23604074
- PMCID: PMC3655472
- DOI: 10.1038/emboj.2013.87
The nascent polypeptide-associated complex is a key regulator of proteostasis
Abstract
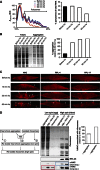
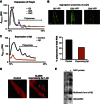
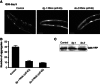
The adaptation of protein synthesis to environmental and physiological challenges is essential for cell viability. Here, we show that translation is tightly linked to the protein-folding environment of the cell through the functional properties of the ribosome bound chaperone NAC (nascent polypeptide-associated complex). Under non-stress conditions, NAC associates with ribosomes to promote translation and protein folding. When proteostasis is imbalanced, NAC relocalizes from a ribosome-associated state to protein aggregates in its role as a chaperone. This results in a functional depletion of NAC from the ribosome that diminishes translational capacity and the flux of nascent proteins. Depletion of NAC from polysomes and re-localisation to protein aggregates is observed during ageing, in response to heat shock and upon expression of the highly aggregation-prone polyglutamine-expansion proteins and Aβ-peptide. These results demonstrate that NAC has a central role as a proteostasis sensor to provide the cell with a regulatory feedback mechanism in which translational activity is also controlled by the folding state of the cellular proteome and the cellular response to stress.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
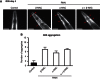
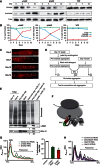
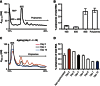
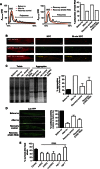

Comment in
-
Ribosome-associated chaperones act as proteostasis sentinels.Cell Cycle. 2013 Aug 1;12(15):2335-6. doi: 10.4161/cc.25703. Epub 2013 Jul 15. Cell Cycle. 2013. PMID: 23856581 Free PMC article. No abstract available.
References
-
- Beatrix B, Sakai H, Wiedmann M (2000) The alpha and beta subunit of the nascent polypeptide-associated complex have distinct functions. J Biol Chem 275: 37838–37845 - PubMed
-
- Bloss TA, Witze ES, Rothman JH (2003) Suppression of CED-3-independent apoptosis by mitochondrial betaNAC in Caenorhabditis elegans. Nature 424: 1066–1071 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

