Inactivation of TGF-β signaling and loss of PTEN cooperate to induce colon cancer in vivo
- PMID: 23604118
- PMCID: PMC3883899
- DOI: 10.1038/onc.2013.102
Inactivation of TGF-β signaling and loss of PTEN cooperate to induce colon cancer in vivo
Abstract
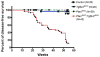
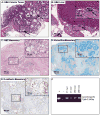
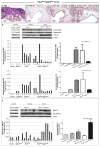
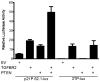
The accumulation of genetic and epigenetic alterations mediates colorectal cancer (CRC) formation by deregulating key signaling pathways in cancer cells. In CRC, one of the most commonly inactivated signaling pathways is the transforming growth factor-beta (TGF-β) signaling pathway, which is often inactivated by mutations of TGF-β type II receptor (TGFBR2). Another commonly deregulated pathway in CRC is the phosphoinositide-3-kinase (PI3K)-AKT pathway. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is an important negative regulator of PI3K-AKT signaling and is silenced in ∼30% of CRC. The combination of TGFBR2 inactivation and loss of PTEN is particularly common in microsatellite-unstable CRCs. Consequently, we determined in vivo if deregulation of these two pathways cooperates to affect CRC formation by analyzing tumors arising in mice that lack Tgfbr2 and/or Pten specifically in the intestinal epithelium. We found that lack of Tgfbr2 (Tgfbr2(IEKO)) alone is not sufficient for intestinal tumor formation and lack of Pten (Pten(IEKO)) alone had a weak effect on intestinal tumor induction. However, the combination of Tgfbr2 inactivation with Pten loss (Pten(IEKO);Tgfbr2(IEKO)) led to malignant tumors in both the small intestine and colon in 86% of the mice and to metastases in 8% of the tumor-bearing mice. Moreover, these tumors arose via a β-catenin-independent mechanism. Inactivation of TGF-β signaling and loss of Pten in the tumors led to increased cell proliferation, decreased apoptosis and decreased expression of cyclin-dependent kinase inhibitors. Thus, inactivation of TGF-β signaling and loss of PTEN cooperate to drive intestinal cancer formation and progression by suppressing cell cycle inhibitors.
Conflict of interest statement
Conflicts of Interest: No conflicts of interest exist for any of the authors.
Figures

Similar articles
-
TGF-β signaling alters the pattern of liver tumorigenesis induced by Pten inactivation.Oncogene. 2015 Jun;34(25):3273-82. doi: 10.1038/onc.2014.258. Epub 2014 Aug 18. Oncogene. 2015. PMID: 25132272 Free PMC article.
-
Transforming growth factor beta receptor type II inactivation induces the malignant transformation of intestinal neoplasms initiated by Apc mutation.Cancer Res. 2006 Oct 15;66(20):9837-44. doi: 10.1158/0008-5472.CAN-06-0890. Cancer Res. 2006. PMID: 17047044
-
TGF-beta receptor inactivation and mutant Kras induce intestinal neoplasms in mice via a beta-catenin-independent pathway.Gastroenterology. 2009 May;136(5):1680-8.e7. doi: 10.1053/j.gastro.2009.01.066. Epub 2009 Feb 4. Gastroenterology. 2009. PMID: 19208363 Free PMC article.
-
Transposon mutagenesis identifies candidate genes that cooperate with loss of transforming growth factor-beta signaling in mouse intestinal neoplasms.Int J Cancer. 2017 Feb 15;140(4):853-863. doi: 10.1002/ijc.30491. Epub 2016 Nov 7. Int J Cancer. 2017. PMID: 27790711 Free PMC article.
-
Tgf-beta signaling alterations and colon cancer.Cancer Treat Res. 2010;155:85-103. doi: 10.1007/978-1-4419-6033-7_5. Cancer Treat Res. 2010. PMID: 20517689 Review.
Cited by
-
A comprehensive analysis of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) loss in colorectal cancer.World J Surg Oncol. 2015 May 20;13:186. doi: 10.1186/s12957-015-0601-y. World J Surg Oncol. 2015. PMID: 25986931 Free PMC article. Clinical Trial.
-
Epithelial-mesenchymal plasticity-engaging stemness in an interplay of phenotypes.Stem Cell Investig. 2019 Aug 20;6:25. doi: 10.21037/sci.2019.08.08. eCollection 2019. Stem Cell Investig. 2019. PMID: 31559312 Free PMC article. Review.
-
Gasdermin C Is Upregulated by Inactivation of Transforming Growth Factor β Receptor Type II in the Presence of Mutated Apc, Promoting Colorectal Cancer Proliferation.PLoS One. 2016 Nov 11;11(11):e0166422. doi: 10.1371/journal.pone.0166422. eCollection 2016. PLoS One. 2016. PMID: 27835699 Free PMC article.
-
The immunomodulatory role of matrix metalloproteinases in colitis-associated cancer.Front Immunol. 2023 Jan 19;13:1093990. doi: 10.3389/fimmu.2022.1093990. eCollection 2022. Front Immunol. 2023. PMID: 36776395 Free PMC article. Review.
-
NF-kappaB-dependent microRNA-425 upregulation promotes gastric cancer cell growth by targeting PTEN upon IL-1β induction.Mol Cancer. 2014 Feb 26;13:40. doi: 10.1186/1476-4598-13-40. Mol Cancer. 2014. PMID: 24571667 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 62(1):10–29. - PubMed
-
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–70. Epub 1996/10/18. - PubMed
-
- Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256(5057):668–70. Epub 1992/05/01. - PubMed
-
- Levy DB, Smith KJ, Beazer-Barclay Y, Hamilton SR, Vogelstein B, Kinzler KW. Inactivation of both APC alleles in human and mouse tumors. Cancer research. 1994;54(22):5953–8. Epub 1994/11/15. - PubMed
-
- Samowitz WS, Powers MD, Spirio LN, Nollet F, van Roy F, Slattery ML. Beta-catenin mutations are more frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer research. 1999;59(7):1442–4. Epub 1999/04/10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

