Monoclonal antibodies toward different Tn-amino acid backbones display distinct recognition patterns on human cancer cells. Implications for effective immuno-targeting of cancer
- PMID: 23604173
- PMCID: PMC11029704
- DOI: 10.1007/s00262-013-1425-7
Monoclonal antibodies toward different Tn-amino acid backbones display distinct recognition patterns on human cancer cells. Implications for effective immuno-targeting of cancer
Abstract
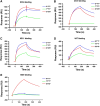
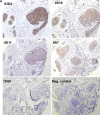
The Tn antigen (GalNAcα-O-Ser/Thr) is a well-established tumor-associated marker which represents a good target for the design of anti-tumor vaccines. Several studies have established that the binding of some anti-Tn antibodies could be affected by the density of Tn determinant or/and by the amino acid residues neighboring O-glycosylation sites. In the present study, using synthetic Tn-based vaccines, we have generated a panel of anti-Tn monoclonal antibodies. Analysis of their binding to various synthetic glycopeptides, modifying the amino acid carrier of the GalNAc(*) (Ser* vs Thr*), showed subtle differences in their fine specificities. We found that the recognition of these glycopeptides by some of these MAbs was strongly affected by the Tn backbone, such as a S*S*S* specific MAb (15G9) which failed to recognize a S*T*T* or a T*T*T* structure. Different binding patterns of these antibodies were also observed in FACS and Western blot analysis using three human cancer cell lines (MCF-7, LS174T and Jurkat). Importantly, an immunohistochemical analysis of human tumors (72 breast cancer and 44 colon cancer) showed the existence of different recognition profiles among the five antibodies evaluated, demonstrating that the aglyconic part of the Tn structure (Ser vs Thr) plays a key role in the anti-Tn specificity for breast and colon cancer detection. This new structural feature of the Tn antigen could be of important clinical value, notably due to the increasing interest of this antigen in anticancer vaccine design as well as for the development of anti-Tn antibodies for in vivo diagnostic and therapeutic strategies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Isolation and characterization of antibodies against three consecutive Tn-antigen clusters from a phage library displaying human single-chain variable fragments.J Biochem. 2010 Jun;147(6):809-17. doi: 10.1093/jb/mvq014. Epub 2010 Feb 10. J Biochem. 2010. PMID: 20147453
-
Analysis of the fine specificity of Tn-binding proteins using synthetic glycopeptide epitopes and a biosensor based on surface plasmon resonance spectroscopy.FEBS Lett. 2000 Mar 3;469(1):24-8. doi: 10.1016/s0014-5793(00)01248-5. FEBS Lett. 2000. PMID: 10708749
-
Molecular basis of antibody binding to mucin glycopeptides in lung cancer.Int J Oncol. 2016 Feb;48(2):587-94. doi: 10.3892/ijo.2015.3302. Epub 2015 Dec 18. Int J Oncol. 2016. PMID: 26692014 Free PMC article.
-
Biotinylated anti-Tn MLS128 monoclonal antibody-streptavidin-111In-DTPA-biotin.2009 Jul 21 [updated 2009 Sep 17]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Jul 21 [updated 2009 Sep 17]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641742 Free Books & Documents. Review.
-
Biotinylated anti-Tn MLS128 monoclonal antibody-125I-streptavidin.2009 Jul 21 [updated 2009 Sep 17]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Jul 21 [updated 2009 Sep 17]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641881 Free Books & Documents. Review.
Cited by
-
The fully synthetic MAG-Tn3 therapeutic vaccine containing the tetanus toxoid-derived TT830-844 universal epitope provides anti-tumor immunity.Cancer Immunol Immunother. 2016 Mar;65(3):315-25. doi: 10.1007/s00262-016-1802-0. Epub 2016 Feb 4. Cancer Immunol Immunother. 2016. PMID: 26847142 Free PMC article.
-
GMMA decorated with mucin 1 Tn/STn mimetics elicit specific antibodies response and inhibit tumor growth.NPJ Vaccines. 2025 Apr 15;10(1):71. doi: 10.1038/s41541-025-01127-8. NPJ Vaccines. 2025. PMID: 40234452 Free PMC article.
-
Mammalian cell-based production of glycans, glycopeptides and glycomodules.Nat Commun. 2024 Nov 8;15(1):9668. doi: 10.1038/s41467-024-53738-9. Nat Commun. 2024. PMID: 39516489 Free PMC article.
-
A Structurally Simple Vaccine Candidate Reduces Progression and Dissemination of Triple-Negative Breast Cancer.iScience. 2020 Jun 26;23(6):101250. doi: 10.1016/j.isci.2020.101250. Epub 2020 Jun 6. iScience. 2020. PMID: 32629615 Free PMC article.
-
Aberrant Glycosylation as Immune Therapeutic Targets for Solid Tumors.Cancers (Basel). 2023 Jul 8;15(14):3536. doi: 10.3390/cancers15143536. Cancers (Basel). 2023. PMID: 37509200 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

