Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits
- PMID: 23604284
- PMCID: PMC3711863
- DOI: 10.1038/nbt.2565
Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits
Abstract
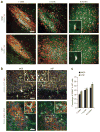
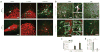
Dysfunction of basal forebrain cholinergic neurons (BFCNs) and γ-aminobutyric acid (GABA) interneurons, derived from medial ganglionic eminence (MGE), is implicated in disorders of learning and memory. Here we present a method for differentiating human embryonic stem cells (hESCs) to a nearly uniform population of NKX2.1(+) MGE-like progenitor cells. After transplantation into the hippocampus of mice in which BFCNs and some GABA neurons in the medial septum had been destroyed by mu P75-saporin, human MGE-like progenitors, but not ventral spinal progenitors, produced BFCNs that synaptically connected with endogenous neurons, whereas both progenitors generated similar populations of GABA neurons. Mice transplanted with MGE-like but not spinal progenitors showed improvements in learning and memory deficits. These results suggest that progeny of the MGE-like progenitors, particularly BFCNs, contributed to learning and memory. Our findings support the prospect of using human stem cell-derived MGE-like progenitors in developing therapies for neurological disorders of learning and memory.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
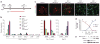



References
-
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. - PubMed
-
- Rubenstein JL, Shimamura K, Martinez S, Puelles L. Regionalization of the prosencephalic neural plate. Annu Rev Neurosci. 1998;21:445–477. - PubMed
-
- Wilson SW, Rubenstein JL. Induction and dorsoventral patterning of the telencephalon. Neuron. 2000;28:641–651. - PubMed
-
- Campbell K. Dorsal-ventral patterning in the mammalian telencephalon. Curr Opin Neurobiol. 2003;13:50–56. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

