TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop
- PMID: 23604321
- PMCID: PMC3699877
- DOI: 10.1038/ncb2718
TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop
Erratum in
- Nat Cell Biol. 2013 Aug;15(8):1016
Abstract
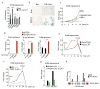
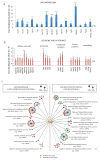
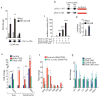
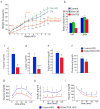
The lysosomal-autophagic pathway is activated by starvation and plays an important role in both cellular clearance and lipid catabolism. However, the transcriptional regulation of this pathway in response to metabolic cues is uncharacterized. Here we show that the transcription factor EB (TFEB), a master regulator of lysosomal biogenesis and autophagy, is induced by starvation through an autoregulatory feedback loop and exerts a global transcriptional control on lipid catabolism via Ppargc1α and Ppar1α. Thus, during starvation a transcriptional mechanism links the autophagic pathway to cellular energy metabolism. The conservation of this mechanism in Caenorhabditis elegans suggests a fundamental role for TFEB in the evolution of the adaptive response to food deprivation. Viral delivery of TFEB to the liver prevented weight gain and metabolic syndrome in both diet-induced and genetic mouse models of obesity, suggesting a new therapeutic strategy for disorders of lipid metabolism.
Conflict of interest statement
The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article at
Figures



Comment in
-
Preventing lysosomal fat indigestion.Nat Cell Biol. 2013 Jun;15(6):565-7. doi: 10.1038/ncb2778. Nat Cell Biol. 2013. PMID: 23728462
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

