A high-throughput method for the quantification of iron saturation in lactoferrin preparations
- PMID: 23604471
- PMCID: PMC3656221
- DOI: 10.1007/s00216-013-6943-9
A high-throughput method for the quantification of iron saturation in lactoferrin preparations
Abstract
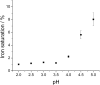
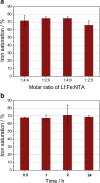
Lactoferrin is considered as a part of the innate immune system that plays a crucial role in preventing bacterial growth, mostly via an iron sequestration mechanism. Recent data show that bovine lactoferrin prevents late-onset sepsis in preterm very low birth weight neonates by serving as an iron chelator for some bacterial strains; thus, it is very important to control the iron saturation level during diet supplementation. An accurate estimation of lactoferrin iron saturation is essential not only because of its clinical applications but also for a wide range of biochemical experiments. A comprehensive method for the quantification of iron saturation in lactoferrin preparations was developed to obtain a calibration curve enabling the determination of iron saturation levels relying exclusively on the defined ratio of absorbances at 280 and 466 nm (A(280/466)). To achieve this goal, selected techniques such as spectrophotometry, ELISA, and ICP-MS were combined. The ability to obtain samples of lactoferrin with determination of its iron content in a simple and fast way has been proven to be very useful. Furthermore, a similar approach could easily be implemented to facilitate the determination of iron saturation level for other metalloproteins in which metal binding results in the appearance of a distinct band in the visible part of the spectrum.
Figures




References
-
- Baker EN (1994) Structure and reactivity of transferrins. In: Sykes AG (ed) Advances in inorganic chemistry, vol. 41. Academic, San Diego, pp 389–463
-
- Aisen P, Leibman A, Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem. 1978;253(6):1930–1937. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

