Central implementation strategies outperform local ones in improving HIV testing in Veterans Healthcare Administration facilities
- PMID: 23605307
- PMCID: PMC3785651
- DOI: 10.1007/s11606-013-2420-6
Central implementation strategies outperform local ones in improving HIV testing in Veterans Healthcare Administration facilities
Abstract
Background: Pilot data suggest that a multifaceted approach may increase HIV testing rates, but the scalability of this approach and the level of support needed for successful implementation remain unknown.
Objective: To evaluate the effectiveness of a scaled-up multi-component intervention in increasing the rate of risk-based and routine HIV diagnostic testing in primary care clinics and the impact of differing levels of program support.
Design: Three arm, quasi-experimental implementation research study.
Setting: Veterans Health Administration (VHA) facilities.
Patients: Persons receiving primary care between June 2009 and September 2011 INTERVENTION: A multimodal program, including a real-time electronic clinical reminder to facilitate HIV testing, provider feedback reports and provider education, was implemented in Central and Local Arm Sites; sites in the Central Arm also received ongoing programmatic support. Control Arm sites had no intervention
Main measures: Frequency of performing HIV testing during the 6 months before and after implementation of a risk-based clinical reminder (phase I) or routine clinical reminder (phase II).
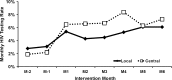
Key results: The adjusted rate of risk-based testing increased by 0.4 %, 5.6 % and 10.1 % in the Control, Local and Central Arms, respectively (all comparisons, p < 0.01). During phase II, the adjusted rate of routine testing increased by 1.1 %, 6.3 % and 9.2 % in the Control, Local and Central Arms, respectively (all comparisons, p < 0.01). At study end, 70-80 % of patients had been offered an HIV test.
Conclusions: Use of clinical reminders, provider feedback, education and social marketing significantly increased the frequency at which HIV testing is offered and performed in VHA facilities. These findings support a multimodal approach toward achieving the goal of having every American know their HIV status as a matter of routine clinical practice.
Figures

References
-
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-infected adults and adolescents. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed February 5, 2013.
-
- Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. - PubMed
-
- Campsmith ML, Rhodes PH, Hall HI, Green TA. Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006. J Acquir Immune Defic Syndr. 2010;53:619–24. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

