MRI confirms loss of blood-brain barrier integrity in a mouse model of disseminated candidiasis
- PMID: 23606437
- PMCID: PMC3744627
- DOI: 10.1002/nbm.2926
MRI confirms loss of blood-brain barrier integrity in a mouse model of disseminated candidiasis
Abstract
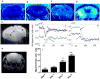
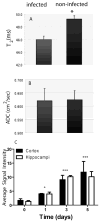
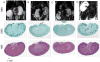
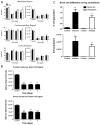
Disseminated candidiasis primarily targets the kidneys and brain in mice and humans. Damage to these critical organs leads to the high mortality associated with such infections, and invasion across the blood-brain barrier can result in fungal meningoencephalitis. Candida albicans can penetrate a brain endothelial cell barrier in vitro through transcellular migration, but this mechanism has not been confirmed in vivo. MRI using the extracellular vascular contrast agent gadolinium diethylenetriaminepentaacetic acid demonstrated that integrity of the blood-brain barrier is lost during C. albicans invasion. Intravital two-photon laser scanning microscopy was used to provide the first real-time demonstration of C. albicans colonizing the living brain, where both yeast and filamentous forms of the pathogen were found. Furthermore, we adapted a previously described method utilizing MRI to monitor inflammatory cell recruitment into infected tissues in mice. Macrophages and other phagocytes were visualized in kidney and brain by the administration of ultrasmall iron oxide particles. In addition to obtaining new insights into the passage of C. albicans across the brain microvasculature, these imaging methods provide useful tools to study further the pathogenesis of C. albicans infections, to define the roles of Candida virulence genes in kidney versus brain infection and to assess new therapeutic measures for drug development.
Keywords: Candida albicans; MRI; blood-brain barrier; extracellular vascular contrast agent Gd-DTPA; intravital two-photon microscopy; murine disseminated candidiasis; ultrasmall iron oxide particles.
Published 2013. This article is a U.S. Government work and is in the public domain in the USA.
Figures


Similar articles
-
Imaging Candida Infections in the Host.Methods Mol Biol. 2016;1356:69-78. doi: 10.1007/978-1-4939-3052-4_6. Methods Mol Biol. 2016. PMID: 26519066 Free PMC article.
-
Candida albicans ISW2 Regulates Chlamydospore Suspensor Cell Formation and Virulence In Vivo in a Mouse Model of Disseminated Candidiasis.PLoS One. 2016 Oct 11;11(10):e0164449. doi: 10.1371/journal.pone.0164449. eCollection 2016. PLoS One. 2016. PMID: 27727302 Free PMC article.
-
Development of Anti-Virulence Approaches for Candidiasis via a Novel Series of Small-Molecule Inhibitors of Candida albicans Filamentation.mBio. 2017 Dec 5;8(6):e01991-17. doi: 10.1128/mBio.01991-17. mBio. 2017. PMID: 29208749 Free PMC article.
-
Investigation of the function of Candida albicans Als3 by heterologous expression in Candida glabrata.Infect Immun. 2013 Jul;81(7):2528-35. doi: 10.1128/IAI.00013-13. Epub 2013 Apr 29. Infect Immun. 2013. PMID: 23630968 Free PMC article.
-
Disruption of the intestinal mucosal barrier in Candida albicans infections.Microbiol Res. 2013 Aug 25;168(7):389-95. doi: 10.1016/j.micres.2013.02.008. Epub 2013 Mar 30. Microbiol Res. 2013. PMID: 23545353 Review.
Cited by
-
It's all in your head: antifungal immunity in the brain.Curr Opin Microbiol. 2020 Dec;58:41-46. doi: 10.1016/j.mib.2020.07.011. Epub 2020 Aug 20. Curr Opin Microbiol. 2020. PMID: 32828989 Free PMC article. Review.
-
Neuroinflammation and structural injury of the fetal ovine brain following intra-amniotic Candida albicans exposure.J Neuroinflammation. 2016 Feb 2;13:29. doi: 10.1186/s12974-016-0492-z. J Neuroinflammation. 2016. PMID: 26842664 Free PMC article.
-
Can oral infection be a risk factor for Alzheimer's disease?J Oral Microbiol. 2015 Sep 17;7:29143. doi: 10.3402/jom.v7.29143. eCollection 2015. J Oral Microbiol. 2015. PMID: 26385886 Free PMC article.
-
Fungal Infection in the Brain: What We Learned from Intravital Imaging.Front Immunol. 2016 Aug 2;7:292. doi: 10.3389/fimmu.2016.00292. eCollection 2016. Front Immunol. 2016. PMID: 27532000 Free PMC article. Review.
-
Could Candida Overgrowth Be Involved in the Pathophysiology of Autism?J Clin Med. 2022 Jan 15;11(2):442. doi: 10.3390/jcm11020442. J Clin Med. 2022. PMID: 35054136 Free PMC article. Review.
References
-
- Kullberg BJ, Filler SG. Candidemia. In: Calderone RA, editor. Candida and Candidiasis. Washington, DC: ASM Press; 2002. pp. 327–340.
-
- Pendlebury WW, Perl DP, Munoz DG. Multiple microabscesses in the central nervous system: a clinicopathologic study. J Neuropathol Exp Neurol. 1989;48:290–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

