A non-inferiority trial of an attenuated combination strategy ('COBRA-light') compared to the original COBRA strategy: clinical results after 26 weeks
- PMID: 23606682
- PMCID: PMC4033113
- DOI: 10.1136/annrheumdis-2012-202818
A non-inferiority trial of an attenuated combination strategy ('COBRA-light') compared to the original COBRA strategy: clinical results after 26 weeks
Abstract
Background: Early, intensive treatment of rheumatoid arthritis (RA) with the combination of (initially high dose) prednisolone, methotrexate and sulfasalazine (COBRA therapy) considerably lowers disease activity and suppresses radiological progression, but is infrequently prescribed in daily practice. Attenuating the COBRA regimen might lessen concerns about side effects, but the efficacy of such strategies is unknown.
Objective: To compare the 'COBRA-light' strategy with only two drugs, comprising a lower dose of prednisolone (starting at 30 mg/day, tapered to 7.5 mg/day in 9 weeks) and methotrexate (escalated to 25 mg/week in 9 weeks) to COBRA therapy (prednisolone 60 mg/day, tapered to 7.5 mg/day in 6 weeks, methotrexate 7.5 mg/week and sulfasalazine 2 g/day).
Method: An open, randomised controlled, non-inferiority trial in 164 patients with early active RA, all treated according to a treat to target strategy.
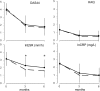
Results: At baseline patients had moderately active disease: mean (SD) 44-joint disease activity score (DAS44) 4.13 (0.81) for COBRA and 3.95 (0.9) for COBRA-light. After 6 months, DAS44 significantly decreased in both groups (-2.50 (1.21) for COBRA and -2.18 (1.10) for COBRA-light). The adjusted difference in DAS44 improvement between the groups, 0.21 (95% CI -0.11 to 0.53), was smaller than the predefined clinically relevant difference of 0.5. Minimal disease activity (DAS44 <1.6) was reached in almost half of patients in both groups (49% and 41% in COBRA and COBRA-light, respectively).
Conclusions: At 6 months COBRA-light therapy is most likely non-inferior to COBRA therapy.
Clinical trial registration number: 55552928.
Keywords: Corticosteroids; DMARDs (synthetic); Early Rheumatoid Arthritis; Treatment.
Figures
References
-
- Korpela M, Laasonen L, Hannonen P, et al. Retardation of joint damage in patients with early rheumatoid arthritis by initial aggressive treatment with disease-modifying antirheumatic drugs: five-year experience from the FIN-RACo study. Arthritis Rheum 2004;50:2072–81 - PubMed
-
- Lard LR, Visser H, Speyer I, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med 2001;111:446–51 - PubMed
-
- Mottonen T, Hannonen P, Leirisalo-Repo M, et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet 1999;353:1568–73 - PubMed
-
- Bakker MF, Jacobs JW, Welsing PM, et al. Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med 2012;156:329–39 - PubMed
-
- Bukhari MA, Wiles NJ, Lunt M, et al. Influence of disease-modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: results from a large observational inception study. Arthritis Rheum 2003;48:46–53 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

