Structure and composition of pulmonary arteries, capillaries, and veins
- PMID: 23606929
- PMCID: PMC3630377
- DOI: 10.1002/cphy.c100081
Structure and composition of pulmonary arteries, capillaries, and veins
Abstract
The pulmonary vasculature comprises three anatomic compartments connected in series: the arterial tree, an extensive capillary bed, and the venular tree. Although, in general, this vasculature is thin-walled, structure is nonetheless complex. Contributions to structure (and thus potentially to function) from cells other than endothelial and smooth muscle cells as well as those from the extracellular matrix should be considered. This review is multifaceted, bringing together information regarding (i) classification of pulmonary vessels, (ii) branching geometry in the pulmonary vascular tree, (iii) a quantitative view of structure based on morphometry of the vascular wall, (iv) the relationship of nerves, a variety of interstitial cells, matrix proteins, and striated myocytes to smooth muscle and endothelium in the vascular wall, (v) heterogeneity within cell populations and between vascular compartments, (vi) homo- and heterotypic cell-cell junctional complexes, and (vii) the relation of the pulmonary vasculature to that of airways. These issues for pulmonary vascular structure are compared, when data is available, across species from human to mouse and shrew. Data from studies utilizing vascular casting, light and electron microscopy, as well as models developed from those data, are discussed. Finally, the need for rigorous quantitative approaches to study of vascular structure in lung is highlighted.
© 2012 American Physiological Society
Figures












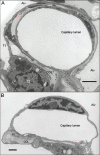





References
-
- Adachi T, Ishikawa K, Hida W, Matsumoto H, Masuda T, Date F, Ogawa K, Takeda K, Furuyama K, Zhang Y, Kitamuro T, Ogawa H, Maruyama Y, Shibahara S. Hypoxemia and blunted hypoxic ventilatory responses in mice lacking heme oxygenase-2. Biochem Biophys Res Commun. 2004;320:514–522. 10.1016/j.bbrc.2004.05.195 S0006291X04012100 [pii] - PubMed
-
- Aguirre JI, Morrell NW, Long L, Clift P, Upton PD, Polak JM, Wilkins MR. Vascular remodeling and ET-1 expression in rat strains with different responses to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2000;278:L981–987. - PubMed
-
- Aharinejad S, Bock P, Lametschwandtner A, Firbas W. Scanning and transmission electron microscopy of venous sphincters in the rat lung. Anat Rec. 1992;233:555–568. 10.1002/ar.1092330410. - PubMed
-
- Aiello VD, Gutierrez PS, Chaves MJ, Lopes AA, Higuchi ML, Ramires JA. Morphology of the internal elastic lamina in arteries from pulmonary hypertensive patients: a confocal laser microscopy study. Mod Pathol. 2003;16:411–416. 10.1097/01.MP.0000067685.57858.D7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

