Ruxolitinib: a potent and selective Janus kinase 1 and 2 inhibitor in patients with myelofibrosis. An update for clinicians
- PMID: 23606937
- PMCID: PMC3627327
- DOI: 10.1177/2040620712459746
Ruxolitinib: a potent and selective Janus kinase 1 and 2 inhibitor in patients with myelofibrosis. An update for clinicians
Abstract
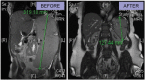
Ruxolitinib became the first US Food and Drug Administration approved therapy for myelofibrosis in 2011 and EU approval is anticipated in summer 2012. Two large phase III trials (known as the COMFORT studies) were the basis for this approval and were published recently. In this review article we discuss the challenges in managing myelofibrosis, the information to date about ruxolitinib and speculate as to the future direction with this and similar agents.
Keywords: JAKafi; janus kinase 2; myelofibrosis; myeloproliferative neoplasm; ruxolitinib.
Conflict of interest statement
Figures
References
-
- Bacigalupo A., Soraru M., Dominietto A., Pozzi S., Geroldi S., Van Lint M., et al. (2010) Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant 45: 458–463 - PubMed
-
- Baffert F., Regnier C., De Pover A., Pissot-Soldermann C., Tavares G., Blasco F., et al. (2010) Potent and selective inhibition of polycythemia by the quinoxaline JAK2 inhibitor NVP-BSK805. Mol Cancer Ther 9: 1945–1955 - PubMed
-
- Barosi G., Birgegard G., Finazzi G., Griesshammer M., Harrison C., Hasselbalch H, et al. (2009) Response criteria for essential thrombocythemia and polycythemia vera: result of a European Leukemianet Consensus Conference. Blood 113: 4829–4833 - PubMed
-
- Barosi G., Mesa R., Thiele J., Cervantes F., Campbell P., Verstovsek S., et al. (2008) Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia 22: 437–438 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials