MR Imaging of Hepatocellular Adenomas and Differential Diagnosis Dilemma
- PMID: 23606972
- PMCID: PMC3623472
- DOI: 10.1155/2013/374170
MR Imaging of Hepatocellular Adenomas and Differential Diagnosis Dilemma
Abstract
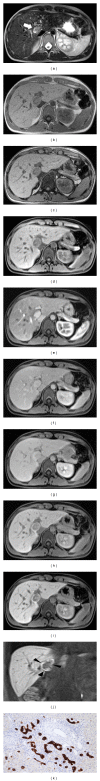
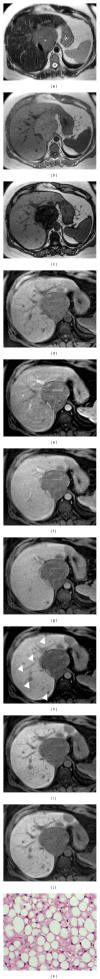
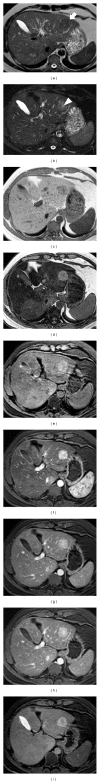
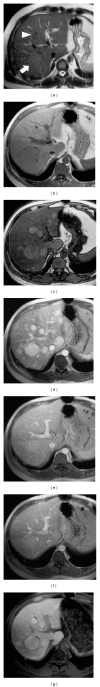
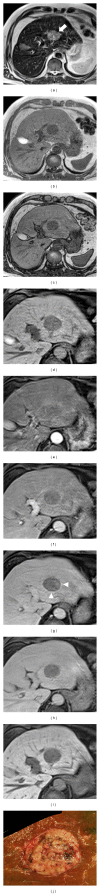
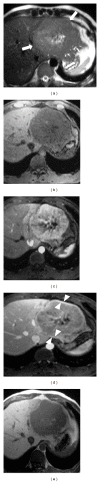
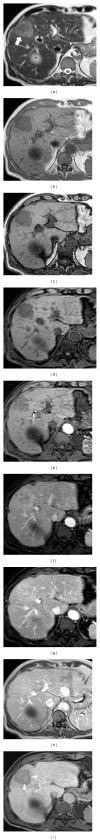
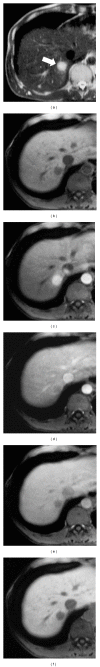
HEPATOCELLULAR ADENOMAS (HCAS) ARE CURRENTLY CATEGORIZED INTO DISTINCT GENETIC AND PATHOLOGIC SUBTYPES AS FOLLOWS: inflammatory hepatocellular adenoma, hepatocyte-nuclear-factor-1-alpha (HNF-1 α -mutated) hepatocellular adenoma, and β -catenin-mutated hepatocellular adenomas; the fourth, defined as unclassified subtype, encompasses HCAs without any genetic abnormalities. This classification has accepted management implications due to different risks of haemorrhage and malignant transformation of the four subtypes. Imaging guided biopsy and/or surgical resection very important in obtaining definitive characterization; nevertheless, MRI with intra-extravascular and hepatobiliary (dual phase) agents, is an important tool not only in differential subtypes definition but even in surveillance with early identification of complications and discovery of some signs of HCA malignant degeneration. Inflammation, abnormal rich vascularisation, peliotic areas, and abundant fatty infiltration are pathologic findings differently present in the HCA subtypes and they may be detected by multiparametric MRI approach. Lesion enlargement and heterogeneity of signal intensity and of contrast enhancement are signs to be considered in malignant transformation. The purpose of this paper is to present the state of the art of MRI in the diagnosis of HCA and subtype characterization, with particular regard to morphologic and functional information available with dual phase contrast agents, and to discuss differential diagnosis with the most common benign and malignant lesions mimicking HCAs.
Figures





References
-
- La Vecchia C, Tavani A. Female hormones and benign liver tumours. Digestive and Liver Disease. 2006;38(8):535–536. - PubMed
-
- Prentice RL. Epidemiologic data on exogenous hormones and hepatocellular carcinoma and selected other cancers. Preventive Medicine. 1991;20(1):38–46. - PubMed
-
- Labrune P, Trioche P, Duvaltier I, Chevalier P, Odièvre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. Journal of Pediatric Gastroenterology and Nutrition. 1997;24(3):276–279. - PubMed
-
- Giannitrapani L, Soresi M, La Spada E, Cervello M, D’Alessandro N, Montalto G. Sex hormones and risk of liver tumor. Annals of the New York Academy of Sciences. 2006;1089:228–236. - PubMed
-
- Cappell MS. Hepatic disorders severely affected by pregnancy: medical and obstetric management. Medical Clinics of North America. 2008;92(4):739–760. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

