Subcutaneous immunoglobulin (16 or 20%) therapy in obese patients with primary immunodeficiency: a retrospective analysis of administration by infusion pump or subcutaneous rapid push
- PMID: 23607310
- PMCID: PMC3722936
- DOI: 10.1111/cei.12099
Subcutaneous immunoglobulin (16 or 20%) therapy in obese patients with primary immunodeficiency: a retrospective analysis of administration by infusion pump or subcutaneous rapid push
Abstract
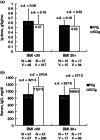
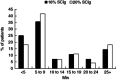
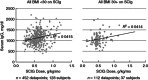
A retrospective chart review was conducted at a single centre, capturing data on 173 primary immunodeficiency disease (PIDD) patients, including 40 obese patients, using subcutaneous administration of immunoglobulin (Ig) (SCIG) (16 or 20%) delivered by infusion pump or subcutaneous (s.c.) rapid push. Patients previously using Ig administered as intravenous (i.v.) infusions (IVIG) were converted to SCIG dosing on a 1:1 basis. In both obese and non-obese patients, mean serum Ig levels were higher during SCIG administration (steady state) compared with IVIG administration (trough values). Similar SCIG dose : serum IgG level relationships were observed between obese and non-obese patients, suggesting the consistent bioavailability of SCIG regardless of body mass index (BMI). The mean SCIG volume per dosing site and the mean number of dosing days per week were greater with s.c. rapid push compared with infusion pump in this cohort, but the mean number of sites per infusion session was lower with s.c. rapid push. Both methods were well tolerated. The use of 20 versus 16% SCIG in obese patients improved dosing efficiency, resulting in smaller weekly volumes (54·7 versus 74·5 ml/week) and dosing on fewer days per week (2·3 versus 3·4 days). These data do not suggest a need for SCIG dosing adjustments in obese individuals relative to non-obese patients. The administration of SCIG using either infusion pump or s.c. rapid push is a practical and well-tolerated alternative to IVIG in obese patients. Offering various administration techniques provides a greater opportunity for treatment satisfaction and patient empowerment, which may support high levels of patient compliance.
Keywords: Hizentra; Vivaglobin; obesity; primary immunodeficiency disease; subcutaneous immunoglobulin therapy.
© 2013 British Society for Immunology.
Figures
References
-
- Berger M, Rojavin M, Kiessling P, Zenker O. Pharmacokinetics of subcutaneous immunoglobulin and their use in dosing of replacement therapy in patients with primary immunodeficiencies. Clin Immunol. 2011;139:133–141. - PubMed
-
- Berger M. Subcutaneous administration of IgG. Immunol Allergy Clin North Am. 2008;28:779–802. - PubMed
-
- Chapel HM, Spickett GP, Ericson D, Engl W, Eibl MM, Bjorkander J. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20:94–100. - PubMed
-
- Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26:265–273. - PubMed
-
- Gardulf A, Nicolay U, Asensio O, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies – a prospective, multi-national study. J Clin Immunol. 2006;26:177–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

