Delayed onset of graft-versus-host disease in immunodeficent human leucocyte antigen-DQ8 transgenic, murine major histocompatibility complex class II-deficient mice repopulated by human peripheral blood mononuclear cells
- PMID: 23607364
- PMCID: PMC3722935
- DOI: 10.1111/cei.12121
Delayed onset of graft-versus-host disease in immunodeficent human leucocyte antigen-DQ8 transgenic, murine major histocompatibility complex class II-deficient mice repopulated by human peripheral blood mononuclear cells
Abstract
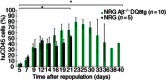
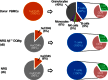
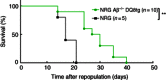
Haematopoietic humanization of mice is used frequently to study the human immune system and its reaction upon experimental intervention. Immunocompromised non-obese diabetic (NOD)-Rag1(-/-) mice, additionally deficient for the common gamma chain of cytokine receptors (γc) (NOD-Rag1(-/-) γc(-/-) mice), lack B, T and natural killer (NK) cells and allow for efficient human peripheral mononuclear cell (PBMC) engraftment. However, a major experimental drawback for studies using these mice is the rapid onset of graft-versus-host disease (GVHD). In order to elucidate the contribution of the xenogenic murine major histocompatibility complex (MHC) class II in this context, we generated immunodeficient mice expressing human MHC class II [human leucocyte antigen (HLA)-DQ8] on a mouse class II-deficient background (Aβ(-/-) ). We studied repopulation and onset of GVHD in these mouse strains following transplantation of DQ8 haplotype-matched human PBMCs. The presence of HLA class II promoted the repopulation rates significantly in these mice. Virtually all the engrafted cells were CD3(+) T cells. The presence of HLA class II did not advance B cell engraftment, such that humoral immune responses were undetectable. However, the overall survival of DQ8-expressing mice was prolonged significantly compared to mice expressing mouse MHC class II molecules, and correlated with an increased time span until onset of GVHD. Our data thus demonstrate that this new mouse strain is useful to study GVHD, and the prolonged animal survival and engraftment rates make it superior for experimental intervention following PBMC engraftment.
Keywords: MHC class II; graft-versus-host disease (GVHD); immunodeficiency-primary.
© 2013 British Society for Immunology.
Figures



Similar articles
-
Lack of acute xenogeneic graft- versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression.FASEB J. 2019 Mar;33(3):3137-3151. doi: 10.1096/fj.201800636R. Epub 2018 Nov 1. FASEB J. 2019. PMID: 30383447 Free PMC article.
-
Development of novel major histocompatibility complex class I and class II-deficient NOD-SCID IL2R gamma chain knockout mice for modeling human xenogeneic graft-versus-host disease.Methods Mol Biol. 2010;602:105-17. doi: 10.1007/978-1-60761-058-8_7. Methods Mol Biol. 2010. PMID: 20012395 Free PMC article.
-
Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex.Clin Exp Immunol. 2009 Jul;157(1):104-18. doi: 10.1111/j.1365-2249.2009.03933.x. Clin Exp Immunol. 2009. PMID: 19659776 Free PMC article.
-
Humanization of Immunodeficient Animals for the Modeling of Transplantation, Graft Versus Host Disease, and Regenerative Medicine.Transplantation. 2020 Nov;104(11):2290-2306. doi: 10.1097/TP.0000000000003177. Transplantation. 2020. PMID: 32068660 Free PMC article. Review.
-
PBMC-engrafted humanized mice models for evaluating immune-related and anticancer drug delivery systems.Front Mol Biosci. 2024 Aug 20;11:1447315. doi: 10.3389/fmolb.2024.1447315. eCollection 2024. Front Mol Biosci. 2024. PMID: 39228913 Free PMC article. Review.
Cited by
-
Xenogeneic Animal Models of Graft-Versus-Host Disease.Methods Mol Biol. 2025;2907:57-70. doi: 10.1007/978-1-0716-4430-0_2. Methods Mol Biol. 2025. PMID: 40100592
-
Progress, implications, and challenges in using humanized immune system mice in CAR-T therapy-Application evaluation and improvement.Animal Model Exp Med. 2024 Feb;7(1):3-11. doi: 10.1002/ame2.12353. Epub 2023 Oct 12. Animal Model Exp Med. 2024. PMID: 37823214 Free PMC article. Review.
-
The Use of a Humanized NSG-β2m-/- Model for Investigation of Immune and Anti-tumor Effects Mediated by the Bifunctional Immunotherapeutic Bintrafusp Alfa.Front Oncol. 2020 Apr 21;10:549. doi: 10.3389/fonc.2020.00549. eCollection 2020. Front Oncol. 2020. PMID: 32373533 Free PMC article.
-
Characterization of a switchable chimeric antigen receptor platform in a pre-clinical solid tumor model.Oncoimmunology. 2017 Jun 20;6(10):e1342909. doi: 10.1080/2162402X.2017.1342909. eCollection 2017. Oncoimmunology. 2017. PMID: 29123951 Free PMC article.
-
Inhibition of HIV Replication by Apolipoprotein A-I Binding Protein Targeting the Lipid Rafts.mBio. 2020 Jan 21;11(1):e02956-19. doi: 10.1128/mBio.02956-19. mBio. 2020. PMID: 31964734 Free PMC article.
References
-
- Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. - PubMed
-
- Lapidot T, Pflumio F, Doedens M, Murdoch B, Williams DE, Dick JE. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992;255:1137–1141. - PubMed
-
- Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. - PubMed
-
- Lieber MR, Hesse JE, Lewis S, et al. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988;55:7–16. - PubMed
-
- Blunt T, Finnie NJ, Taccioli GE, et al. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813–823. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

