Anti-tumour necrosis factor therapy enhances mucosal healing through down-regulation of interleukin-21 expression and T helper type 17 cell infiltration in Crohn's disease
- PMID: 23607532
- PMCID: PMC3694540
- DOI: 10.1111/cei.12084
Anti-tumour necrosis factor therapy enhances mucosal healing through down-regulation of interleukin-21 expression and T helper type 17 cell infiltration in Crohn's disease
Abstract
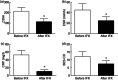
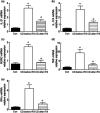
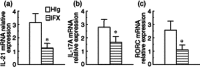
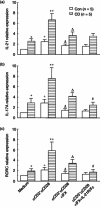
Anti-tumour necrosis factor (TNF) monoclonal antibody (mAb) (infliximab, IFX) has been shown to be highly effective in the management of Crohn's disease (CD). Herein we investigated the potential role of IFX in inducing clinical remission and regulating interleukin (IL)-21 expression and T helper type 17 (Th17) cell infiltration in the intestinal mucosa of CD patients. Twenty-six CD patients were treated with IFX at weeks 0, 2 and 6. Clinical response, mucosal healing, serum C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were evaluated at week 10 after IFX administration. Expression of IL-21, IL-17A and retinoic acid-related orphan receptor C (RORC) in intestinal mucosa were analysed by quantitative real-time polymerase chain reaction (PCR) and immunohistochemistry. Peripheral blood and lamina propria CD4(+) T cells were stimulated with anti-CD3 and anti-CD28 mAbs in the presence of IFX. Cytokine profiles and RORC were determined with enzyme-linked immunosorbent assay (ELISA) and real-time PCR. IL-21 and Th17 cells were found to be expressed highly in inflamed mucosa of active CD patients compared with healthy controls. Ten weeks after IFX infusion, CD activity index, ESR, CRP and intestinal mucosal healing were improved markedly in CD patients, and IL-21 expression and Th17 cell infiltration were decreased significantly compared with those before IFX therapy. In-vitro study demonstrated that IFX treatment could suppress IL-21, IL-17A and RORC expression in cultured CD biopsies. Moreover, IFX was also observed to down-regulate markedly IL-17A, IL-21 and RORC expression by CD CD4(+) T cells. IFX is highly effective in inducing clinical remission and promoting intestinal mucosal healing in CD patients through down-regulation of IL-21 expression and Th17 cell infiltration in intestinal mucosa.
© 2013 British Society for Immunology.
Figures

Similar articles
-
Anti-TNF Therapy Induces CD4+ T-Cell Production of IL-22 and Promotes Epithelial Repairs in Patients With Crohn's Disease.Inflamm Bowel Dis. 2018 Jul 12;24(8):1733-1744. doi: 10.1093/ibd/izy126. Inflamm Bowel Dis. 2018. PMID: 29718341
-
IL-25 downregulates Th1/Th17 immune response in an IL-10-dependent manner in inflammatory bowel disease.Inflamm Bowel Dis. 2013 Mar-Apr;19(4):720-8. doi: 10.1097/MIB.0b013e3182802a76. Inflamm Bowel Dis. 2013. PMID: 23429464
-
Reduction of CD68+ macrophages and decreased IL-17 expression in intestinal mucosa of patients with inflammatory bowel disease strongly correlate with endoscopic response and mucosal healing following infliximab therapy.Inflamm Bowel Dis. 2013 Mar-Apr;19(4):729-39. doi: 10.1097/MIB.0b013e318280292b. Inflamm Bowel Dis. 2013. PMID: 23448791
-
Role of the IL23/IL17 Pathway in Crohn's Disease.Front Immunol. 2021 Mar 30;12:622934. doi: 10.3389/fimmu.2021.622934. eCollection 2021. Front Immunol. 2021. PMID: 33859636 Free PMC article. Review.
-
The role of Th17 cytokines in primary mucosal immunity.Cytokine Growth Factor Rev. 2010 Dec;21(6):443-8. doi: 10.1016/j.cytogfr.2010.11.002. Epub 2010 Nov 20. Cytokine Growth Factor Rev. 2010. PMID: 21095154 Free PMC article. Review.
Cited by
-
Infliximab preferentially induces clinical remission and mucosal healing in short course Crohn's disease with luminal lesions through balancing abnormal immune response in gut mucosa.Mediators Inflamm. 2015;2015:793764. doi: 10.1155/2015/793764. Epub 2015 Mar 19. Mediators Inflamm. 2015. PMID: 25873771 Free PMC article.
-
Ectopic Tertiary Lymphoid Tissue in Inflammatory Bowel Disease: Protective or Provocateur?Front Immunol. 2016 Aug 16;7:308. doi: 10.3389/fimmu.2016.00308. eCollection 2016. Front Immunol. 2016. PMID: 27579025 Free PMC article. Review.
-
Evaluating IL-21 as a Potential Therapeutic Target in Crohn's Disease.Gastroenterol Res Pract. 2018 Apr 10;2018:5962624. doi: 10.1155/2018/5962624. eCollection 2018. Gastroenterol Res Pract. 2018. PMID: 29849593 Free PMC article.
-
Capturing the Biologic Onset of Inflammatory Bowel Diseases: Impact on Translational and Clinical Science.Cells. 2019 Jun 6;8(6):548. doi: 10.3390/cells8060548. Cells. 2019. PMID: 31174359 Free PMC article. Review.
-
Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases.World J Gastroenterol. 2016 Nov 14;22(42):9300-9313. doi: 10.3748/wjg.v22.i42.9300. World J Gastroenterol. 2016. PMID: 27895418 Free PMC article. Review.
References
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
-
- Di Sabatino A, Biancheri P, Rovedatti L, et al. New pathogenic paradigms in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:368–371. - PubMed
-
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

