A radical transfer pathway in spore photoproduct lyase
- PMID: 23607538
- PMCID: PMC3666868
- DOI: 10.1021/bi3016247
A radical transfer pathway in spore photoproduct lyase
Erratum in
- Biochemistry. 2013 Jul 16;52(28):4869
Abstract
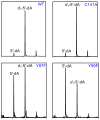
Spore photoproduct lyase (SPL) repairs a covalent UV-induced thymine dimer, spore photoproduct (SP), in germinating endospores and is responsible for the strong UV resistance of endospores. SPL is a radical S-adenosyl-l-methionine (SAM) enzyme, which uses a [4Fe-4S](+) cluster to reduce SAM, generating a catalytic 5'-deoxyadenosyl radical (5'-dA(•)). This in turn abstracts a H atom from SP, generating an SP radical that undergoes β scission to form a repaired 5'-thymine and a 3'-thymine allylic radical. Recent biochemical and structural data suggest that a conserved cysteine donates a H atom to the thymine radical, resulting in a putative thiyl radical. Here we present structural and biochemical data that suggest that two conserved tyrosines are also critical in enzyme catalysis. One [Y99(Bs) in Bacillus subtilis SPL] is downstream of the cysteine, suggesting that SPL uses a novel hydrogen atom transfer (HAT) pathway with a pair of cysteine and tyrosine residues to regenerate SAM. The other tyrosine [Y97(Bs)] has a structural role to facilitate SAM binding; it may also contribute to the SAM regeneration process by interacting with the putative (•)Y99(Bs) and/or 5'-dA(•) intermediates to lower the energy barrier for the second H abstraction step. Our results indicate that SPL is the first member of the radical SAM superfamily (comprising more than 44000 members) to bear a catalytically operating HAT chain.
Figures
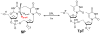




Similar articles
-
Mechanistic studies of the radical SAM enzyme spore photoproduct lyase (SPL).Biochim Biophys Acta. 2012 Nov;1824(11):1264-77. doi: 10.1016/j.bbapap.2011.11.008. Epub 2011 Dec 8. Biochim Biophys Acta. 2012. PMID: 22197590 Free PMC article. Review.
-
The enzyme-mediated direct reversal of a dithymine photoproduct in germinating endospores.Int J Mol Sci. 2013 Jun 25;14(7):13137-53. doi: 10.3390/ijms140713137. Int J Mol Sci. 2013. PMID: 23799365 Free PMC article.
-
Mechanistic studies of the spore photoproduct lyase via a single cysteine mutation.Biochemistry. 2012 Sep 11;51(36):7173-88. doi: 10.1021/bi3010945. Epub 2012 Aug 31. Biochemistry. 2012. PMID: 22906093 Free PMC article.
-
Characterization of an active spore photoproduct lyase, a DNA repair enzyme in the radical S-adenosylmethionine superfamily.J Biol Chem. 2006 Sep 8;281(36):25994-6003. doi: 10.1074/jbc.M603931200. Epub 2006 Jul 7. J Biol Chem. 2006. PMID: 16829680
-
Spore photoproduct lyase: the known, the controversial, and the unknown.J Biol Chem. 2015 Feb 13;290(7):4003-9. doi: 10.1074/jbc.R114.573675. Epub 2014 Dec 4. J Biol Chem. 2015. PMID: 25477522 Free PMC article. Review.
Cited by
-
Properties of Site-Specifically Incorporated 3-Aminotyrosine in Proteins To Study Redox-Active Tyrosines: Escherichia coli Ribonucleotide Reductase as a Paradigm.Biochemistry. 2018 Jun 19;57(24):3402-3415. doi: 10.1021/acs.biochem.8b00160. Epub 2018 Apr 17. Biochemistry. 2018. PMID: 29630358 Free PMC article.
-
Kinetic Isotope Effects and Hydrogen/Deuterium Exchange Reveal Large Conformational Changes During the Catalysis of the Clostridium acetobutylicum Spore Photoproduct Lyase.Photochem Photobiol. 2017 Jan;93(1):331-342. doi: 10.1111/php.12697. Epub 2017 Jan 30. Photochem Photobiol. 2017. PMID: 27992649 Free PMC article.
-
Radical SAM Enzyme Spore Photoproduct Lyase: Properties of the Ω Organometallic Intermediate and Identification of Stable Protein Radicals Formed during Substrate-Free Turnover.J Am Chem Soc. 2020 Oct 28;142(43):18652-18660. doi: 10.1021/jacs.0c08585. Epub 2020 Oct 15. J Am Chem Soc. 2020. PMID: 32966073 Free PMC article.
-
Pathways of thymidine hypermodification.Nucleic Acids Res. 2022 Apr 8;50(6):3001-3017. doi: 10.1093/nar/gkab781. Nucleic Acids Res. 2022. PMID: 34522950 Free PMC article.
-
Radical SAM Enzymes and Ribosomally-Synthesized and Post-translationally Modified Peptides: A Growing Importance in the Microbiomes.Front Chem. 2021 Jul 19;9:678068. doi: 10.3389/fchem.2021.678068. eCollection 2021. Front Chem. 2021. PMID: 34350157 Free PMC article. Review.
References
-
- Setlow P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. 2006;101:514–525. - PubMed
-
- Desnous CL, Guillaume D, Clivio P. Spore photoproduct: A key to bacterial eternal life. Chem Rev. 2010;110:1213–1232. - PubMed
-
- Fajardo-Cavazos P, Rebeil R, Nicholson W. Essential cysteine residues in Bacillus subtilis spore photoproduct lyase identified by alanine scanning mutagenesis. Curr Microbiol. 2005;51:331–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

