A radical transfer pathway in spore photoproduct lyase
- PMID: 23607538
- PMCID: PMC3666868
- DOI: 10.1021/bi3016247
A radical transfer pathway in spore photoproduct lyase
Erratum in
- Biochemistry. 2013 Jul 16;52(28):4869
Abstract
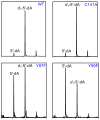
Spore photoproduct lyase (SPL) repairs a covalent UV-induced thymine dimer, spore photoproduct (SP), in germinating endospores and is responsible for the strong UV resistance of endospores. SPL is a radical S-adenosyl-l-methionine (SAM) enzyme, which uses a [4Fe-4S](+) cluster to reduce SAM, generating a catalytic 5'-deoxyadenosyl radical (5'-dA(•)). This in turn abstracts a H atom from SP, generating an SP radical that undergoes β scission to form a repaired 5'-thymine and a 3'-thymine allylic radical. Recent biochemical and structural data suggest that a conserved cysteine donates a H atom to the thymine radical, resulting in a putative thiyl radical. Here we present structural and biochemical data that suggest that two conserved tyrosines are also critical in enzyme catalysis. One [Y99(Bs) in Bacillus subtilis SPL] is downstream of the cysteine, suggesting that SPL uses a novel hydrogen atom transfer (HAT) pathway with a pair of cysteine and tyrosine residues to regenerate SAM. The other tyrosine [Y97(Bs)] has a structural role to facilitate SAM binding; it may also contribute to the SAM regeneration process by interacting with the putative (•)Y99(Bs) and/or 5'-dA(•) intermediates to lower the energy barrier for the second H abstraction step. Our results indicate that SPL is the first member of the radical SAM superfamily (comprising more than 44000 members) to bear a catalytically operating HAT chain.
Figures
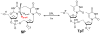




References
-
- Setlow P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. 2006;101:514–525. - PubMed
-
- Desnous CL, Guillaume D, Clivio P. Spore photoproduct: A key to bacterial eternal life. Chem Rev. 2010;110:1213–1232. - PubMed
-
- Fajardo-Cavazos P, Rebeil R, Nicholson W. Essential cysteine residues in Bacillus subtilis spore photoproduct lyase identified by alanine scanning mutagenesis. Curr Microbiol. 2005;51:331–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

