Enantioselective redox-relay oxidative heck arylations of acyclic alkenyl alcohols using boronic acids
- PMID: 23607624
- PMCID: PMC3698857
- DOI: 10.1021/ja402916z
Enantioselective redox-relay oxidative heck arylations of acyclic alkenyl alcohols using boronic acids
Abstract
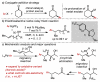
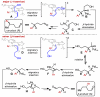
A general, highly selective asymmetric redox-relay oxidative Heck reaction using achiral or racemic acyclic alkenols and boronic acid derivatives is reported. This reaction delivers remotely functionalized arylated carbonyl products from acyclic alkenol substrates, with excellent enantioselectivity under mild conditions, bearing a range of useful functionality. A preliminary mechanistic investigation suggests that the regioselectivity of the initial migratory insertion is highly dependent on the electronic nature of the boronic acid and more subtle electronic effects of the alkenyl alcohol.
Figures
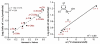
References
-
-
For selected reviews, see: Sun Y-W, Zhu P-L, Xu Q, Shi M. RSC Adv. 2013;3:3153. Hawner C, Alexakis A. Chem. Commun. 2010;46:7295. Harutyunyan SR, den Hartog T, Geurts K, Minnaard AJ, Feringa BL. Chem. Rev. 2008;108:2824. Hayashi T, Yamasaki K. Chem. Rev. 2003;103:2829.
-
-
-
For the selected reviews, see: Mc Cartney D, Guiry P. J. Chem. Soc. Rev. 2011;40:5122. Shibasaki M, Vogl EM, Ohshima T. Adv. Synth. Catal. 2004;346:1533. Dounay AB, Overman LE. Chem. Rev. 2003;103:2945.
-
-
-
For an early example of intermolecular asymmetric Heck reaction with acyclic olefins, see: Yonehara K, Mori K, Hashizume T, Chung K-G, Ohe K, Uemura S. J. Organomet. Chem. 2000;603:40.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

