Investigation of the cutaneous response to recall antigen in humans in vivo
- PMID: 23607634
- PMCID: PMC3722916
- DOI: 10.1111/cei.12107
Investigation of the cutaneous response to recall antigen in humans in vivo
Abstract
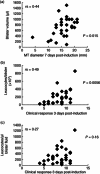
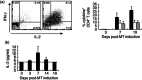
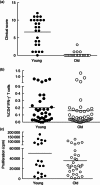
In this paper we provide a detailed description of an experimental method for investigating the induction and resolution of recall immune response to antigen in humans in vivo. This involves the injection of tuberculin purified protein derivative (PPD) into the skin, followed by inducing suction blisters at the site of injection, from which leucocytes and cytokines that are involved in the response can be isolated and characterized. Using this technique we found that although the majority of CD4(+) T cells in the skin that are present early in the response express cutaneous lymphocyte antigen (CLA), the expression of this marker is reduced significantly in later phases. This may enable these cells to leave the skin during immune resolution. Furthermore, interleukin (IL)-2 production can be detected both in CD4(+) T cells and also in the blister fluid at the peak of the response at day 7, indicating that mediators found in the blister fluid are representative of the cytokine microenvironment in vivo. Finally, we found that older humans have defective ability to respond to cutaneous PPD challenge, but this does not reflect a global immune deficit as they have similar numbers of circulating functional PPD-specific CD4(+) T cells as young subjects. The use of the blister technology enables further characterization of the skin specific defect in older humans and also general mechanisms that govern immune regulation in vivo.
Keywords: DTH; ageing; human immunity.
© 2013 British Society for Immunology.
Figures



References
-
- Hayday AC, Peakman M. The habitual, diverse and surmountable obstacles to human immunology research. Nat Immunol. 2008;9:575–580. - PubMed
-
- Vukmanovic-Stejic M, Reed JR, Lacy KE, Rustin MH, Akbar AN. Mantoux test as a model for a secondary immune response in humans. Immunol Lett. 2006;107:93–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

