Mesenchymal stem cells (MSCs) from scleroderma patients (SSc) preserve their immunomodulatory properties although senescent and normally induce T regulatory cells (Tregs) with a functional phenotype: implications for cellular-based therapy
- PMID: 23607751
- PMCID: PMC3722920
- DOI: 10.1111/cei.12111
Mesenchymal stem cells (MSCs) from scleroderma patients (SSc) preserve their immunomodulatory properties although senescent and normally induce T regulatory cells (Tregs) with a functional phenotype: implications for cellular-based therapy
Abstract
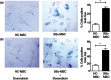
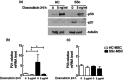
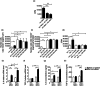
Systemic sclerosis (SSc) is a chronic disease, with early activation of the immune system. The aim of our work was to address how SSc-mesenchymal stem cells (MSCs), although senescent, might preserve specific immunomodulatory abilities during SSc. MSCs were obtained from 10 SSc patients and 10 healthy controls (HC). Senescence was evaluated by assessing cell cycle, β-galactosidase (β-Gal) activity, p21 and p53 expression; doxorubicin was used as acute senescence stimulus to evaluate their ability to react in stressed conditions. Immunomodulatory abilities were studied co-culturing MSCs with peripheral blood mononuclear cells (PBMCs) and CD4(+) cells, in order to establish both their ability to block proliferation in mixed lymphocyte reaction and in regulatory T cells (Tregs) induction. SSc-MSC showed an increase of senescence biomarkers. Eighty per cent of MSCs were in G0-G1 phase, without significant differences between SSc and HC. SSc-MSCs showed an increased positive β-Gal staining and higher p21 transcript level compared to HC cells. After doxorubicin, β-Gal staining increased significantly in SSc-MSCs. On the contrary, doxorubicin abolished p21 activation and elicited p53 induction both in SSc- and HC-MSCs. Interleukin (IL)-6 and transforming growth factor (TGF)-β-related transcripts and their protein levels were significantly higher in SSc-MSCs. The latter maintained their immunosuppressive effect on lymphocyte proliferation and induced a functionally regulatory phenotype on T cells, increasing surface expression of CD69 and restoring the regulatory function which is impaired in SSc. Increased activation of the IL-6 pathway observed in our cells might represent an adaptive mechanism to senescence, but preserving some specific cellular functions, including immunosuppression.
Keywords: immunomodulatory abilities; mesenchymal stem cells; senescence; systemic sclerosis.
© 2013 British Society for Immunology.
Figures

References
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Le Blanc K, Frassoni F, Ball L, et al. Developmental Committee of the European Group for Blood and Marrow transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a Phase II study. Lancet. 2008;371:1579–1586. - PubMed
-
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. - PubMed
-
- Oswald J, Boxberger S, Jørgensen B, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

