Immunophenotyping and protein profiling of Fontan-associated plastic bronchitis airway casts
- PMID: 23607837
- PMCID: PMC3960899
- DOI: 10.1513/AnnalsATS.201209-080OC
Immunophenotyping and protein profiling of Fontan-associated plastic bronchitis airway casts
Abstract
Rationale: Plastic bronchitis (PB) is a rare and deadly condition that is characterized by the formation of airway casts. It most frequently occurs in children with underlying congenital heart disease that has been surgically palliated by the Fontan procedure. The Fontan circulation results in above-normal central venous pressure, and it has been hypothesized that the formation of airway casts is due to lymph leak. Knowledge of plastic bronchitis pathogenesis is poor and stems mostly from published case reports.
Objectives: To garner information about cast pathogenesis by characterizing inflammatory cell phenotypes in existing formalin-preserved, paraffin-embedded samples and generating protein and cytokine-chemokine profiles of airway cast homogenates.
Methods: We used immunofluorescence confocal microscopy, state-of-the-science proteomics, and a cytokine array assay to immunophenotype cellular content and to generate protein and cytokine profiles of plastic bronchitis airway casts, respectively.
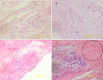
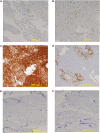
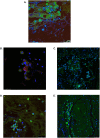
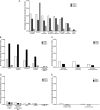
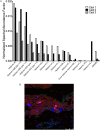
Measurements and main results: Neutrophils, eosinophils, macrophages, and B lymphocytes were identified in cast samples; there were notably fewer T lymphocytes. Fibrin(ogen) was an abundant protein in the cast proteome. Histone H4 was also abundant, and immunofluorescence microscopy demonstrated it to be mostly extracellular. The cytokine profile of plastic bronchitis casts was proinflammatory.
Conclusions: Plastic bronchitis airway casts from children with Fontan physiology are composed of fibrin and are cellular and inflammatory in nature, providing evidence that their formation cannot be explained simply by lymph leak into the airways. Consequences of cellular necrosis including extracellular histones and the apparent low number of T cells indicate that a derangement in inflammation resolution likely contributes to cast formation.
Figures

References
-
- Healy F, Hanna BD, Zinman R. Pulmonary complications of congenital heart disease. Paediatr Respir Rev. 2012;13:10–15. - PubMed
-
- Goldberg DJ, Dodds K, Rychik J. Rare problems associated with the Fontan circulation. Cardiol Young. 2010;20:113–119. - PubMed
-
- Madsen P, Shah SA, Rubin BK. Plastic bronchitis: new insights and a classification scheme. Paediatr Respir Rev. 2005;6:292–300. - PubMed
-
- Seear M, Hui H, Magee F, Bohn D, Cutz E. Bronchial casts in children: a proposed classification based on nine cases and a review of the literature. Am J Respir Crit Care Med. 1997;155:364–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

