Differential type 1 interferon-regulated gene expression in the brain during AIDS: interactions with viral diversity and neurovirulence
- PMID: 23608145
- PMCID: PMC3955194
- DOI: 10.1096/fj.13-227868
Differential type 1 interferon-regulated gene expression in the brain during AIDS: interactions with viral diversity and neurovirulence
Abstract
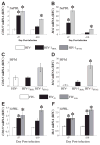
The lentiviruses, human and feline immunodeficiency viruses (HIV-1 and FIV, respectively), infect the brain and cause neurovirulence, evident as neuronal injury, inflammation, and neurobehavioral abnormalities with diminished survival. Herein, different lentivirus infections in conjunction with neural cell viability were investigated, concentrating on type 1 interferon-regulated pathways. Transcriptomic network analyses showed a preponderance of genes involved in type 1 interferon signaling, which was verified by increased expression of the type 1 interferon-associated genes, Mx1 and CD317, in brains from HIV-infected persons (P<0.05). Leukocytes infected with different strains of FIV or HIV-1 showed differential Mx1 and CD317 expression (P<0.05). In vivo studies of animals infected with the FIV strains, FIV(ch) or FIV(ncsu), revealed that FIV(ch)-infected animals displayed deficits in memory and motor speed compared with the FIV(ncsu)- and mock-infected groups (P<0.05). TNF-α, IL-1β, and CD40 expression was increased in the brains of FIV(ch)-infected animals; conversely, Mx1 and CD317 transcript levels were increased in the brains of FIV(ncsu)-infected animals, principally in microglia (P<0.05). Gliosis and neuronal loss were evident among FIV(ch)-infected animals compared with mock- and FIV(ncsu)-infected animals (P<0.05). Lentiviral infections induce type 1 interferon-regulated gene expression in microglia in a viral diversity-dependent manner, representing a mechanism by which immune responses might be exploited to limit neurovirulence.
Keywords: BST-2; CD317; FIV; HIV-1; microglia; tetherin; type 1 interferon.
Figures


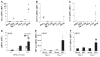
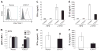
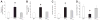
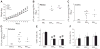
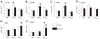

Similar articles
-
Neurosteroid-mediated regulation of brain innate immunity in HIV/AIDS: DHEA-S suppresses neurovirulence.FASEB J. 2013 Feb;27(2):725-37. doi: 10.1096/fj.12-215079. Epub 2012 Nov 12. FASEB J. 2013. PMID: 23150523
-
Regulation of lentivirus neurovirulence by lipopolysaccharide conditioning: suppression of CXCL10 in the brain by IL-10.J Immunol. 2010 Feb 1;184(3):1566-74. doi: 10.4049/jimmunol.0902575. Epub 2009 Dec 30. J Immunol. 2010. PMID: 20042580
-
Feline tetherin efficiently restricts release of feline immunodeficiency virus but not spreading of infection.J Virol. 2011 Jun;85(12):5840-52. doi: 10.1128/JVI.00071-11. Epub 2011 Apr 13. J Virol. 2011. PMID: 21490095 Free PMC article.
-
Neurologic disease in feline immunodeficiency virus infection: disease mechanisms and therapeutic interventions for NeuroAIDS.J Neurovirol. 2018 Apr;24(2):220-228. doi: 10.1007/s13365-017-0593-1. Epub 2017 Dec 15. J Neurovirol. 2018. PMID: 29247305 Review.
-
Comparative neurovirulence in lentiviral infections: The roles of viral molecular diversity and select proteases.J Neurovirol. 2004;10 Suppl 1:113-7. doi: 10.1080/753312762. J Neurovirol. 2004. PMID: 14982749 Review.
Cited by
-
Gene Expression: the Key to Understanding HIV-1 Infection?Microbiol Mol Biol Rev. 2020 May 13;84(2):e00080-19. doi: 10.1128/MMBR.00080-19. Print 2020 May 20. Microbiol Mol Biol Rev. 2020. PMID: 32404327 Free PMC article. Review.
-
HIV life cycle, innate immunity and autophagy in the central nervous system.Curr Opin HIV AIDS. 2014 Nov;9(6):565-71. doi: 10.1097/COH.0000000000000106. Curr Opin HIV AIDS. 2014. PMID: 25203639 Free PMC article. Review.
-
Human Interleukin-34 facilitates microglia-like cell differentiation and persistent HIV-1 infection in humanized mice.Mol Neurodegener. 2019 Mar 5;14(1):12. doi: 10.1186/s13024-019-0311-y. Mol Neurodegener. 2019. PMID: 30832693 Free PMC article.
-
Transcriptomic Analysis of Age-Associated Periventricular Lesions Reveals Dysregulation of the Immune Response.Int J Mol Sci. 2020 Oct 25;21(21):7924. doi: 10.3390/ijms21217924. Int J Mol Sci. 2020. PMID: 33113879 Free PMC article.
-
Inflammasome induction in Rasmussen's encephalitis: cortical and associated white matter pathogenesis.J Neuroinflammation. 2013 Dec 13;10:152. doi: 10.1186/1742-2094-10-152. J Neuroinflammation. 2013. PMID: 24330827 Free PMC article.
References
-
- Zink MC, Laast VA, Helke KL, Brice AK, Barber SA, Clements JE, Mankowski JL. From mice to macaques–animal models of HIV nervous system disease. Curr HIV Res. 2006;4:293–305. - PubMed
-
- Power C. Retroviral diseases of the nervous system: pathogenic host response or viral gene-mediated neurovirulence? Trends Neurosci. 2001;24:162–169. - PubMed
-
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

