IL-4 inhibition of IL-1 induced Matrix metalloproteinase-3 (MMP-3) expression in human fibroblasts involves decreased AP-1 activation via negative crosstalk involving of Jun N-terminal kinase (JNK)
- PMID: 23608488
- PMCID: PMC3757497
- DOI: 10.1016/j.yexcr.2013.04.010
IL-4 inhibition of IL-1 induced Matrix metalloproteinase-3 (MMP-3) expression in human fibroblasts involves decreased AP-1 activation via negative crosstalk involving of Jun N-terminal kinase (JNK)
Abstract
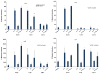
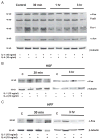
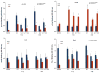
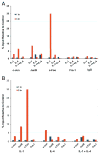
Matrix metalloproteinase-3 (MMP-3) over-expression is associated with tissue destruction in the context of chronic inflammation. Previous studies showed that IL-4 inhibits induction of MMP-3 by IL-1β, and suggested that AP-1 might be involved. Here we show that IL-1 induced binding of transcription factor AP-1 to the MMP-3 promoter consists primarily of c-Jun, JunB, and c-Fos and that binding of c-Jun and c-Fos is inhibited by the combination of cytokines while binding of Jun B is not. Mutation of the AP-1 site in the MMP-3 promoter decreased the ability of IL-4 to inhibit its transcription in transfected MG-63 cells. Western blotting showed that both cytokines activate Jun N-terminal kinase (JNK), but with somewhat different kinetics, and that activation of JNK by both cytokines individually is inhibited by the combination. These results indicate that IL-4 inhibition of MMP-3 expression is associated with reduction of IL-1 induced binding of active forms of the AP-1 dimer, while less active JunB-containing dimers remain, and suggest that these changes are associated with decreased activation of JNK.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Honig J, Rordorf-Adam C, Siegmund C, Wiedemann W, Erard F, et al. Increased interleukin-1 beta (IL-1 beta) concentration in gingival tissue from periodontitis patients. J Periodontal Res. 1989;24:362–367. - PubMed
-
- Stashenko P, Fujiyoshi P, Obernesser MS, Prostak L, Haffajee AD, Socransky SS, et al. Levels of interleukin 1 beta in tissue from sites of active periodontal disease. J Clin Periodontol. 1991;18:548–554. - PubMed
-
- Cheung NT, Dawes PT, Poulton KV, Ollier WE, Taylor DJ, Mattey DL, et al. High serum levels of pro-matrix metalloproteinase-3 are associated with greater radiographic damage and the presence of the shared epitope in patients with rheumatoid arthritis. J Rheumatol. 2000;27:882–887. - PubMed
-
- van den Berg WB, Bresnihan B, et al. Pathogenesis of joint damage in rheumatoid arthritis: evidence of a dominant role for interleukin-I. Baillieres Best Pract Res Clin Rheumatol. 1999;13:577–597. - PubMed
-
- Shapira L, van Dyke TE, Hart TC, et al. A localized absence of interleukin-4 triggers periodontal disease activity: a novel hypothesis. Med Hypotheses. 1992;39:319–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

