Delineation of the key aspects in the regulation of epithelial monolayer formation
- PMID: 23608536
- PMCID: PMC3700122
- DOI: 10.1128/MCB.01435-12
Delineation of the key aspects in the regulation of epithelial monolayer formation
Abstract
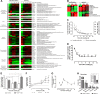
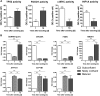
The formation, maintenance, and repair of epithelial barriers are of critical importance for whole-body homeostasis. However, the molecular events involved in epithelial tissue maturation are not fully established. To this end, we investigated the molecular processes involved in renal epithelial proximal-tubule monolayer maturation utilizing transcriptomic, metabolomic, and functional parameters. We uncovered profound dynamic alterations in transcriptional regulation, energy metabolism, and nutrient utilization over the maturation process. Proliferating cells exhibited high glycolytic rates and high transcript levels for fatty acid synthesis genes (FASN), whereas matured cells had low glycolytic rates, increased oxidative capacity, and preferentially expressed genes for beta oxidation. There were dynamic alterations in the expression and localization of several adherens (CDH1, -4, and -16) and tight junction (TJP3 and CLDN2 and -10) proteins. Genes involved in differentiated proximal-tubule function, cilium biogenesis (BBS1), and transport (ATP1A1 and ATP1B1) exhibited increased expression during epithelial maturation. Using TransAM transcription factor activity assays, we could demonstrate that p53 and FOXO1 were highly active in matured cells, whereas HIF1A and c-MYC were highly active in proliferating cells. The data presented here will be invaluable in the further delineation of the complex dynamic cellular processes involved in epithelial cell regulation.
Figures







References
-
- Jennings P, Koppelstaetter C, Lechner J, Pfaller W. 2008. Renal culture models: contribution to the understanding of nephrotoxic mechanisms, p. 223–250 In Broe ME, Porter GA. (ed), Clinical nephrotoxins: renal injury from drugs and chemicals, 3rd ed Springer, New York, NY
-
- Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M. 2010. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc. Natl. Acad. Sci. U. S. A. 107: 8011–8016 - PMC - PubMed
-
- Gstraunthaler G, Pfaller W, Kotanko P. 1985. Interrelation between oxygen consumption and Na-K-ATPase activity in rat renal proximal tubule suspension. Ren. Physiol. 8: 38–44 - PubMed
-
- Jennings P. 2009. Epithelial cell cultures as tools to study human disease and the adverse effects of pharmaceuticals and chemicals. Habilitation thesis Medizinische Universität Innsbruck, Innsbruck, Austria
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
