Tumor uptake of hollow gold nanospheres after intravenous and intra-arterial injection: PET/CT study in a rabbit VX2 liver cancer model
- PMID: 23608932
- PMCID: PMC4164961
- DOI: 10.1007/s11307-013-0635-x
Tumor uptake of hollow gold nanospheres after intravenous and intra-arterial injection: PET/CT study in a rabbit VX2 liver cancer model
Abstract
Purpose: This study was designed to investigate the intratumoral uptake of hollow gold nanospheres (HAuNS) after hepatic intra-arterial (IA) and intravenous (IV) injection in a liver tumor model.
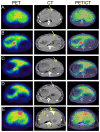
Materials and methods: Fifteen VX2 tumor-bearing rabbits were randomized into five groups (n = 3 in each group) that received either IV (64)Cu-labeled PEG-HAuNS (IV-PEG-HAuNS), IA (64)Cu-labeled PEG-HAuNS (IA-PEG-HAuNS), IV cyclic peptide (RGD)-conjugated (64)Cu-labeled PEG-HAuNS (IV-RGD-PEG-HAuNS), IA RGD-conjugated (64)Cu-labeled PEG-HAuNS (IA-RGD-PEG-HAuNS), or IA (64)Cu-labeled PEG-HAuNS with lipiodol (IA-PEG-HAuNS-lipiodol). The animals underwent PET/CT 1 h after injection, and uptake expressed as percentage of injected dose per gram of tissue (%ID/g) was measured in tumor and major organs. The animals were euthanized 24 h after injection, and tissues were evaluated for radioactivity.
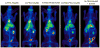
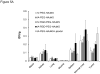
Results: At 1 h after injection, animals in the IA-PEG-HAuNS-lipiodol group showed significantly higher tumor uptake (P < 0.001) and higher ratios of tumor-to-normal liver uptake (P < 0.001) than those in all other groups. The biodistribution of radioactivity 24 h after injection showed that IA delivery of PEG-HAuNS with lipiodol resulted in the highest tumor uptake (0.33 %ID/g; P < 0.001) and tumor-to-normal liver ratio (P < 0.001) among all delivery methods. At 24 h, the IA-RGD-PEG-HAuNS group showed higher tumor uptake than the IA-PEG-HAuNS group (0.20 vs. 0.099 %ID/g; P < 0.001).
Conclusion: Adding iodized oil to IA-PEG-HAuNS maximizes nanoparticle delivery to hepatic tumors and therefore may be useful in targeted chemotherapy and photoablative therapy. PET/CT can be used to noninvasively monitor the biodistribution of radiolabeled HAuNS after IV or IA injection.
Figures





References
-
- Hong K, Georgiades CS, Geschwind JF. Technology insight: Image-guided therapies for hepatocellular carcinoma--intra-arterial and ablative techniques. Nat Clin Pract Oncol. 2006;3:315–324. - PubMed
-
- Ridge JA, Collin C, Bading JR, Hancock C, Conti PS, Daly JM, Raaf JH. Increased adriamycin levels in hepatic implants of rabbit Vx-2 carcinoma from regional infusion. Cancer Res. 1988;48:4584–4587. - PubMed
-
- Farrell D, Ptak K, Panaro NJ, Grodzinski P. Nanotechnology-Based Cancer Therapeutics-Promise and Challenge-Lessons Learned Through the NCI Alliance for Nanotechnology in Cancer. Pharm Res. 2010;28:273–278. - PubMed
-
- Maeng JH, Lee DH, Jung KH, Bae YH, Park IS, Jeong S, Jeon YS, Shim CK, Kim W, Kim J, Lee J, Lee YM, Kim JH, Kim WH, Hong SS. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials. 2010;31:4995–5006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

