Biogenic fish-gut calcium carbonate is a stable amorphous phase in the gilt-head seabream, Sparus aurata
- PMID: 23609008
- PMCID: PMC3632881
- DOI: 10.1038/srep01700
Biogenic fish-gut calcium carbonate is a stable amorphous phase in the gilt-head seabream, Sparus aurata
Abstract
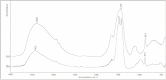
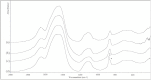
The main source of calcium carbonate (CaCO₃) in the ocean comes from the shells of calcifying planktonic organisms, but substantial amounts of CaCO₃ are also produced in fish intestines. The precipitation of CaCO₃ assists fish in intestinal water absorption and aids in whole body Ca²⁺ homeostasis. Here we report that the product formed in the intestinal lumen of the gilt-head seabream, Sparus aurata, is an amorphous calcium carbonate (ACC) phase. With FTIR spectroscopy and SEM imaging, our study shows that the fish-derived carbonates from S. aurata are maintained as a stable amorphous phase throughout the intestinal tract. Moreover, intestinal deposits contained up to 54 mol% Mg²⁺, the highest concentration yet reported in biogenic ACC. Mg is most likely responsible for stabilizing this inherently unstable mineral. The fish carbonates also displayed initial rapid dissolution when exposed to seawater, exhibiting a significant increase in carbonate concentration.
Figures


Similar articles
-
Water absorption and bicarbonate secretion in the intestine of the sea bream are regulated by transmembrane and soluble adenylyl cyclase stimulation.J Comp Physiol B. 2012 Dec;182(8):1069-80. doi: 10.1007/s00360-012-0685-4. Epub 2012 Jun 30. J Comp Physiol B. 2012. PMID: 22752677
-
Increased intestinal carbonate precipitate abundance in the sea bream (Sparus aurata L.) in response to ocean acidification.PLoS One. 2019 Jun 21;14(6):e0218473. doi: 10.1371/journal.pone.0218473. eCollection 2019. PLoS One. 2019. PMID: 31226164 Free PMC article.
-
Amorphous calcium carbonate: A precursor phase for aragonite in shell disease of the pearl oyster.Biochem Biophys Res Commun. 2018 Feb 26;497(1):102-107. doi: 10.1016/j.bbrc.2018.02.031. Epub 2018 Feb 8. Biochem Biophys Res Commun. 2018. PMID: 29428728
-
Biologically formed amorphous calcium carbonate.Connect Tissue Res. 2003;44 Suppl 1:214-8. Connect Tissue Res. 2003. PMID: 12952200 Review.
-
Intestinal bicarbonate secretion by marine teleost fish--why and how?Biochim Biophys Acta. 2002 Nov 13;1566(1-2):182-93. doi: 10.1016/s0005-2736(02)00600-4. Biochim Biophys Acta. 2002. PMID: 12421549 Review.
Cited by
-
Innovation in Osteogenesis Activation: Role of Marine-Derived Materials in Bone Regeneration.Curr Issues Mol Biol. 2025 Mar 7;47(3):175. doi: 10.3390/cimb47030175. Curr Issues Mol Biol. 2025. PMID: 40136429 Free PMC article. Review.
-
Temperature, species identity and morphological traits predict carbonate excretion and mineralogy in tropical reef fishes.Nat Commun. 2023 Feb 22;14(1):985. doi: 10.1038/s41467-023-36617-7. Nat Commun. 2023. PMID: 36813767 Free PMC article.
-
Osmoregulation by the gastro-intestinal tract of marine fish at depth - implications for the global carbon cycle.J Exp Biol. 2025 Jul 15;228(14):jeb249834. doi: 10.1242/jeb.249834. Epub 2025 Jul 18. J Exp Biol. 2025. PMID: 40679146 Free PMC article.
-
The Kinetics of Aragonite Formation from Solution via Amorphous Calcium Carbonate.Nanomaterials (Basel). 2022 Nov 23;12(23):4151. doi: 10.3390/nano12234151. Nanomaterials (Basel). 2022. PMID: 36500773 Free PMC article.
-
Bioinspired Stabilization of Amorphous Calcium Carbonate by Carboxylated Nanocellulose Enables Mechanically Robust, Healable, and Sensing Biocomposites.ACS Nano. 2023 Apr 11;17(7):6664-6674. doi: 10.1021/acsnano.2c12385. Epub 2023 Mar 22. ACS Nano. 2023. PMID: 36946540 Free PMC article.
References
-
- Morse J. W., Arvidson R. S. & Luttge A. Calcium carbonate formation and dissolution. Chem. Rev. 107, 342–381 (2007). - PubMed
-
- Kleypas J. A. & Langdon C. Coral reefs and changing seawater chemistry. In: Coral Reefs and Climate Change: Science and Management, Coastal and Estuarine Studies (J. T. Phinney, O. Hoegh-Guldberg, J. Kleypas, W. Skirving, A. Strong Eds.), 61, American Geophysical Union, Washington, DC (2006), pp. 73–110.
-
- Fabry V. J., Seibel B. A., Feely R. A. & Orr J. C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414 (2008).
-
- Walsh P. J., Blackwelder P., Gill K. A., Danulat E. & Mommsen T. P. Carbonate deposits in marine fish intestines: a new source of biomineralization. Limnol. Oceanogr. 36, 1227–1232 (1991).
-
- Wilson R. W. et al. Contribution of fish to the marine inorganic carbon cycle. Science. 323, 359–362 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

