Prebiotically plausible oligoribonucleotide ligation facilitated by chemoselective acetylation
- PMID: 23609088
- PMCID: PMC4074891
- DOI: 10.1038/nchem.1626
Prebiotically plausible oligoribonucleotide ligation facilitated by chemoselective acetylation
Abstract
The recent synthesis of pyrimidine ribonucleoside-2',3'-cyclic phosphates under prebiotically plausible conditions has strengthened the case for the involvement of ribonucleic acid (RNA) at an early stage in the origin of life. However, a prebiotic conversion of these weakly activated monomers, and their purine counterparts, to the 3',5'-linked RNA polymers of extant biochemistry has been lacking (previous attempts led only to short oligomers with mixed linkages). Here we show that the 2'-hydroxyl group of oligoribonucleotide-3'-phosphates can be chemoselectively acetylated in water under prebiotically credible conditions, which allows rapid and efficient template-directed ligation. The 2'-O-acetyl group at the ligation junction of the product RNA strand can be removed under conditions that leave the internucleotide bonds intact. Remarkably, acetylation of mixed oligomers that possess either 2'- or 3'-terminal phosphates is selective for the 2'-hydroxyl group of the latter. This newly discovered chemistry thus suggests a prebiotic route from ribonucleoside-2',3'-cyclic phosphates to predominantly 3',5'-linked RNA via partially 2'-O-acetylated RNA.
Figures

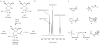




Comment in
-
Chemical origins of life: Prebiotic RNA unstuck.Nat Chem. 2013 May;5(5):360-2. doi: 10.1038/nchem.1636. Nat Chem. 2013. PMID: 23609081 No abstract available.
References
-
- Joyce GF. The antiquity of RNA-based evolution. Nature. 2002;418:214–221. - PubMed
-
- Woese C. The genetic code. Harper & Row; New York: 1967. pp. 179–195.
-
- Crick FHC. The origin of the genetic code. J. Mol. Biol. 1968;38:367–379. - PubMed
-
- Orgel LE. Evolution of the genetic apparatus. J. Mol. Biol. 1968;38:381–393. - PubMed
-
- Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459:239–242. - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/162009983
- PubChem-Substance/162009984
- PubChem-Substance/162009985
- PubChem-Substance/162009986
- PubChem-Substance/162009987
- PubChem-Substance/162009988
- PubChem-Substance/162009989
- PubChem-Substance/162009990
- PubChem-Substance/162009991
- PubChem-Substance/162009992
- PubChem-Substance/162009993
- PubChem-Substance/162009994
- PubChem-Substance/162009995
- PubChem-Substance/162009996
- PubChem-Substance/162009997
- PubChem-Substance/162009998
- PubChem-Substance/162009999
- PubChem-Substance/162010000
- PubChem-Substance/162010001
- PubChem-Substance/162010002
- PubChem-Substance/162010003
- PubChem-Substance/162010004
- PubChem-Substance/162010005
- PubChem-Substance/162010006
- PubChem-Substance/162010007
- PubChem-Substance/162010008
- PubChem-Substance/162023993
- PubChem-Substance/162023994
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

