The Scaffold attachment factor b1 (Safb1) regulates myogenic differentiation by facilitating the transition of myogenic gene chromatin from a repressed to an activated state
- PMID: 23609547
- PMCID: PMC3675494
- DOI: 10.1093/nar/gkt285
The Scaffold attachment factor b1 (Safb1) regulates myogenic differentiation by facilitating the transition of myogenic gene chromatin from a repressed to an activated state
Abstract
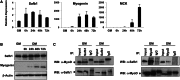
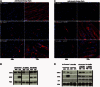
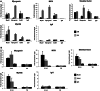
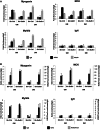
The regulation of skeletal muscle gene expression during myogenesis is mediated by lineage-specific transcription factors in combination with numerous cofactors, many of which modify chromatin structure. However, the involvement of scaffolding proteins that organize chromatin and chromatin-associated regulatory proteins has not extensively been explored in myogenic differentiation. Here, we report that Scaffold attachment factor b1 (Safb1), primarily associated with transcriptional repression, functions as a positive regulator of myogenic differentiation. Knockdown of Safb1 inhibited skeletal muscle marker gene expression and differentiation in cultured C2C12 myoblasts. In contrast, over-expression resulted in the premature expression of critical muscle structural proteins and formation of enlarged thickened myotubes. Safb1 co-immunoprecipitated with MyoD and was co-localized on myogenic promoters. Upon Safb1 knockdown, the repressive H3K27me3 histone mark and binding of the Polycomb histone methyltransferase Ezh2 persisted at differentiation-dependent gene promoters. In contrast, the appearance of histone marks and regulators associated with myogenic gene activation, such as myogenin and the SWI/SNF chromatin remodelling enzyme ATPase, Brg1, was blocked. These results indicate that the scaffold protein Safb1 contributes to the activation of skeletal muscle gene expression during myogenic differentiation by facilitating the transition of promoter sequences from a repressive chromatin structure to one that is transcriptionally permissive.
Figures


Similar articles
-
Myogenin and the SWI/SNF ATPase Brg1 maintain myogenic gene expression at different stages of skeletal myogenesis.J Biol Chem. 2007 Mar 2;282(9):6564-70. doi: 10.1074/jbc.M608898200. Epub 2006 Dec 27. J Biol Chem. 2007. PMID: 17194702
-
Ppp1r1b-lncRNA inhibits PRC2 at myogenic regulatory genes to promote cardiac and skeletal muscle development in mouse and human.RNA. 2020 Apr;26(4):481-491. doi: 10.1261/rna.073692.119. Epub 2020 Jan 17. RNA. 2020. PMID: 31953255 Free PMC article.
-
The myogenic basic helix-loop-helix family of transcription factors shows similar requirements for SWI/SNF chromatin remodeling enzymes during muscle differentiation in culture.J Biol Chem. 2002 Sep 13;277(37):33818-24. doi: 10.1074/jbc.M205159200. Epub 2002 Jul 8. J Biol Chem. 2002. PMID: 12105204
-
Temporal regulation of chromatin during myoblast differentiation.Semin Cell Dev Biol. 2017 Dec;72:77-86. doi: 10.1016/j.semcdb.2017.10.022. Epub 2017 Oct 28. Semin Cell Dev Biol. 2017. PMID: 29079444 Free PMC article. Review.
-
SWI/SNF complexes, chromatin remodeling and skeletal myogenesis: it's time to exchange!Exp Cell Res. 2010 Nov 1;316(18):3073-80. doi: 10.1016/j.yexcr.2010.05.023. Epub 2010 May 27. Exp Cell Res. 2010. PMID: 20553711 Free PMC article. Review.
Cited by
-
Long noncoding RNA Neat1 modulates myogenesis by recruiting Ezh2.Cell Death Dis. 2019 Jun 26;10(7):505. doi: 10.1038/s41419-019-1742-7. Cell Death Dis. 2019. PMID: 31243262 Free PMC article.
-
Differential requirements for different subfamilies of the mammalian SWI/SNF chromatin remodeling enzymes in myoblast cell cycle progression and expression of the Pax7 regulator.Biochim Biophys Acta Gene Regul Mech. 2022 Feb;1865(2):194801. doi: 10.1016/j.bbagrm.2022.194801. Epub 2022 Feb 23. Biochim Biophys Acta Gene Regul Mech. 2022. PMID: 35217218 Free PMC article.
-
Differential Contributions of mSWI/SNF Chromatin Remodeler Sub-Families to Myoblast Differentiation.Int J Mol Sci. 2023 Jul 9;24(14):11256. doi: 10.3390/ijms241411256. Int J Mol Sci. 2023. PMID: 37511016 Free PMC article.
-
Chromatin Landscape During Skeletal Muscle Differentiation.Front Genet. 2020 Sep 18;11:578712. doi: 10.3389/fgene.2020.578712. eCollection 2020. Front Genet. 2020. PMID: 33193700 Free PMC article. Review.
-
The chromatin scaffold protein SAFB1 localizes SUMO-1 to the promoters of ribosomal protein genes to facilitate transcription initiation and splicing.Nucleic Acids Res. 2015 Apr 20;43(7):3605-13. doi: 10.1093/nar/gkv246. Epub 2015 Mar 23. Nucleic Acids Res. 2015. PMID: 25800734 Free PMC article.
References
-
- Perdiguero E, Sousa-Victor P, Ballestar E, Munoz-Canoves P. Epigenetic regulation of myogenesis. Epigenetics. 2009;4:541–550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

