Polydopamine-based surface modification for the development of peritumorally activatable nanoparticles
- PMID: 23609560
- PMCID: PMC3700573
- DOI: 10.1007/s11095-013-1039-y
Polydopamine-based surface modification for the development of peritumorally activatable nanoparticles
Abstract
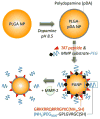
Purpose: To create poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs), where a drug-encapsulating NP core is covered with polyethylene glycol (PEG) in a normal condition but exposes a cell-interactive TAT-modified surface in an environment rich in matrix metalloproteinases (MMPs).
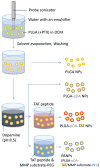
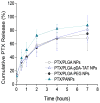
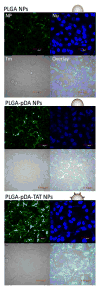
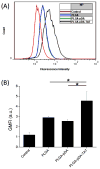
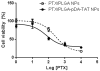
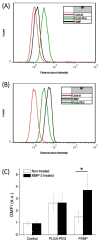
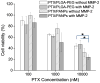
Methods: PLGA NPs were modified with TAT peptide (PLGA-pDA-TAT NPs) or dual-modified with TAT peptide and a conjugate of PEG and MMP-substrate peptide (peritumorally activatable NPs, PANPs) via dopamine polymerization. Cellular uptake of fluorescently labeled NPs was observed with or without a pre-treatment of MMP-2 by confocal microscopy and flow cytometry. NPs loaded with paclitaxel (PTX) were tested against SKOV-3 ovarian cancer cells to evaluate the contribution of surface modification to cellular delivery of PTX.
Results: While the size and morphology did not significantly change due to the modification, NPs modified with dopamine polymerization were recognized by their dark color. TAT-containing NPs (PLGA-pDA-TAT NPs and PANPs) showed changes in surface charge, indicative of effective conjugation of TAT peptide on the surface. PLGA-pDA-TAT NPs and MMP-2-pre-treated PANPs showed relatively good cellular uptake compared to PLGA NPs, MMP-2-non-treated PANPs, and NPs with non-cleavable PEG. After 3 h treatment with cells, PTX loaded in cell-interactive NPs showed greater toxicity than non-interactive ones as the former could enter cells during the incubation period. However, due to the initial burst drug release, the difference was not as clear as microscopic observation.
Conclusions: PEGylated polymeric NPs that could expose cell-interactive surface in response to MMP-2 were successfully created by dual modification of PLGA NPs using dopamine polymerization.
Figures


Similar articles
-
Synthesis, characterization, and evaluation of paclitaxel loaded in six-arm star-shaped poly(lactic-co-glycolic acid).Int J Nanomedicine. 2013;8:4315-26. doi: 10.2147/IJN.S51629. Epub 2013 Nov 7. Int J Nanomedicine. 2013. PMID: 24235829 Free PMC article.
-
Folate-receptor-targeted laser-activable poly(lactide-co-glycolic acid) nanoparticles loaded with paclitaxel/indocyanine green for photoacoustic/ultrasound imaging and chemo/photothermal therapy.Int J Nanomedicine. 2018 Sep 6;13:5139-5158. doi: 10.2147/IJN.S167043. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30233177 Free PMC article.
-
Beyond the imaging: limitations of cellular uptake study in the evaluation of nanoparticles.J Control Release. 2012 Dec 10;164(2):170-6. doi: 10.1016/j.jconrel.2012.04.042. Epub 2012 May 5. J Control Release. 2012. PMID: 22568932 Free PMC article.
-
Biomedical applications of PLGA nanoparticles in nanomedicine: advances in drug delivery systems and cancer therapy.Expert Opin Drug Deliv. 2023 Jul-Dec;20(7):937-954. doi: 10.1080/17425247.2023.2223941. Epub 2023 Jun 23. Expert Opin Drug Deliv. 2023. PMID: 37294853 Review.
-
Enabling anticancer therapeutics by nanoparticle carriers: the delivery of Paclitaxel.Int J Mol Sci. 2011;12(7):4395-413. doi: 10.3390/ijms12074395. Epub 2011 Jul 7. Int J Mol Sci. 2011. PMID: 21845085 Free PMC article. Review.
Cited by
-
Modifications of natural peptides for nanoparticle and drug design.Adv Protein Chem Struct Biol. 2015;98:57-91. doi: 10.1016/bs.apcsb.2014.12.001. Epub 2015 Mar 12. Adv Protein Chem Struct Biol. 2015. PMID: 25819276 Free PMC article. Review.
-
Cytotoxicity of targeted PLGA nanoparticles: a systematic review.RSC Adv. 2021 Mar 3;11(16):9433-9449. doi: 10.1039/d1ra00074h. eCollection 2021 Mar 1. RSC Adv. 2021. PMID: 35423427 Free PMC article. Review.
-
Extracellularly activatable nanocarriers for drug delivery to tumors.Expert Opin Drug Deliv. 2014 Oct;11(10):1601-1618. doi: 10.1517/17425247.2014.930434. Epub 2014 Jun 20. Expert Opin Drug Deliv. 2014. PMID: 24950343 Free PMC article. Review.
-
Cellular Uptake Mechanism of Paclitaxel Nanocrystals Determined by Confocal Imaging and Kinetic Measurement.AAPS J. 2015 Sep;17(5):1126-34. doi: 10.1208/s12248-015-9774-0. Epub 2015 Jun 24. AAPS J. 2015. PMID: 26104805 Free PMC article.
-
Intraperitoneal chemotherapy of ovarian cancer by hydrogel depot of paclitaxel nanocrystals.J Control Release. 2016 Aug 10;235:91-98. doi: 10.1016/j.jconrel.2016.05.056. Epub 2016 May 26. J Control Release. 2016. PMID: 27238443 Free PMC article.
References
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12):6387–92. - PubMed
-
- Gabizon AA, Shmeeda H, Zalipsky S. Pros and cons of the liposome platform in cancer drug targeting. J Liposome Res. 2006;16(3):175–83. - PubMed
-
- Yokoyama M. Drug targeting with nano-sized carrier systems. J Artif Organs. 2005;8(2):77–84. - PubMed
-
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60. - PubMed
-
- Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 62(2):90–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

