Progress in gene therapy for neurological disorders
- PMID: 23609618
- PMCID: PMC3908892
- DOI: 10.1038/nrneurol.2013.56
Progress in gene therapy for neurological disorders
Erratum in
- Nat Rev Neurol. 2013 Jun;9(6):298
Abstract
Diseases of the nervous system have devastating effects and are widely distributed among the population, being especially prevalent in the elderly. These diseases are often caused by inherited genetic mutations that result in abnormal nervous system development, neurodegeneration, or impaired neuronal function. Other causes of neurological diseases include genetic and epigenetic changes induced by environmental insults, injury, disease-related events or inflammatory processes. Standard medical and surgical practice has not proved effective in curing or treating these diseases, and appropriate pharmaceuticals do not exist or are insufficient to slow disease progression. Gene therapy is emerging as a powerful approach with potential to treat and even cure some of the most common diseases of the nervous system. Gene therapy for neurological diseases has been made possible through progress in understanding the underlying disease mechanisms, particularly those involving sensory neurons, and also by improvement of gene vector design, therapeutic gene selection, and methods of delivery. Progress in the field has renewed our optimism for gene therapy as a treatment modality that can be used by neurologists, ophthalmologists and neurosurgeons. In this Review, we describe the promising gene therapy strategies that have the potential to treat patients with neurological diseases and discuss prospects for future development of gene therapy.
Figures

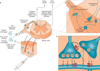
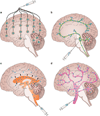

References
-
- Elsabahy M, Nazarali A, Foldvari M. Non-viral nucleic acid delivery: key challenges and future directions. Curr. Drug Deliv. 2011;8:235–244. - PubMed
Publication types
MeSH terms
Grants and funding
- 1NS054193/NS/NINDS NIH HHS/United States
- R01 NS061107/NS/NINDS NIH HHS/United States
- CA119298/CA/NCI NIH HHS/United States
- R24 EY019861/EY/NEI NIH HHS/United States
- R21 NS084275/NS/NINDS NIH HHS/United States
- NS038850/NS/NINDS NIH HHS/United States
- DK044935/DK/NIDDK NIH HHS/United States
- R01 NS056243/NS/NINDS NIH HHS/United States
- P30 DK047757/DK/NIDDK NIH HHS/United States
- EY019861/EY/NEI NIH HHS/United States
- NS052465‑04S1/NS/NINDS NIH HHS/United States
- NS069378/NS/NINDS NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- UL1 TR000433/TR/NCATS NIH HHS/United States
- R01 NS054193/NS/NINDS NIH HHS/United States
- OD010939/OD/NIH HHS/United States
- R01 CA119298/CA/NCI NIH HHS/United States
- NS056243/NS/NINDS NIH HHS/United States
- U01 NS069378/NS/NINDS NIH HHS/United States
- R01 NS082311/NS/NINDS NIH HHS/United States
- R01 NS038850/NS/NINDS NIH HHS/United States
- NS057711/NS/NINDS NIH HHS/United States
- R01 NS074387/NS/NINDS NIH HHS/United States
- NS061107/NS/NINDS NIH HHS/United States
- TR000003/TR/NCATS NIH HHS/United States
- U01 NS052465/NS/NINDS NIH HHS/United States
- DK063973/DK/NIDDK NIH HHS/United States
- NS038690/NS/NINDS NIH HHS/United States
- NS052465/NS/NINDS NIH HHS/United States
- NS40923/NS/NINDS NIH HHS/United States
- P01 NS040923/NS/NINDS NIH HHS/United States
- R01 NS038690/NS/NINDS NIH HHS/United States
- DK047757/DK/NIDDK NIH HHS/United States
- NS029390/NS/NINDS NIH HHS/United States
- R01 DK063973/DK/NIDDK NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- DP1 EY023177/EY/NEI NIH HHS/United States
- EY023177/EY/NEI NIH HHS/United States
- NS074387/NS/NINDS NIH HHS/United States
- R01 NS029390/NS/NINDS NIH HHS/United States
- R01 NS057711/NS/NINDS NIH HHS/United States
- P01 DK044935/DK/NIDDK NIH HHS/United States
- TR000433/TR/NCATS NIH HHS/United States
- P40 OD010939/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

