HLA-restricted NY-ESO-1 peptide immunotherapy for metastatic castration resistant prostate cancer
- PMID: 23609828
- PMCID: PMC4100683
- DOI: 10.1007/s10637-013-9960-9
HLA-restricted NY-ESO-1 peptide immunotherapy for metastatic castration resistant prostate cancer
Abstract
Background: Given the immunogenicity of NY-ESO-1 peptides in prostate cancer, a phase I clinical trial was designed to evaluate HLA class-I and class-II restricted NY-ESO-1 peptides in metastatic castration-resistant prostate cancer (mCRPC).
Methods: Patients with progressive mCRPC, Zubrod Performance Status ≤2, PSA ≥10 ng/ml who had appropriate HLA class I (A2) and class II haplotypes (DR4, DP4) were eligible. Three groups with 3 patients each received the vaccine subcutaneously every 2 weeks for 6 doses. Group 1 received a peptide presented by an HLA class I haplotype (HLA-A2), Group 2 with a peptide presented by HLA class II haplotype (DR4, DP4), and Group 3 with peptides presented by both Class I and II haplotypes. Androgen-deprivation was continued. Owing to a myocardial infarction, the protocol was amended to omit the use of GM-CSF.
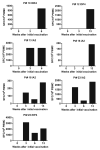
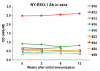
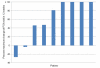
Results: Fourteen patients were evaluable for toxicities and 9 received all 6 doses and were evaluable for efficacy. One death from myocardial infarction following GM-CSF occurred in a patient with generalized myalgias. After omitting GM-CSF, no grade >2 toxicities were observed. Among 9 patients evaluable for efficacy, the median PSA doubling time pre-therapy and during therapy were 3.1 and 4.92 months, respectively. NY-ESO-1 specific T-cell response observed by ELISPOT appeared more frequent in docetaxel-naïve patients (4 of 4) than docetaxel-pretreated patients (2 of 5).
Conclusion: In men with mCRPC, individualized HLA class-I and/or class-II restricted NY-ESO-1 peptides were tolerable, appeared to slow PSA doubling time and yielded antigen-specific T-cell responses more often in chemonaïve patients.
Trial registration: ClinicalTrials.gov NCT00711334.
Figures
References
-
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immuno-therapy for castration-resistant prostate cancer. N Engl J Med. 363:411–22. - PubMed
-
- Jungbluth AA, Chen YT, Stockert E, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer J Int Cancer. 2001;92:856–860. - PubMed
-
- Odunsi K, Jungbluth AA, Stockert E, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immuno-therapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–6083. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

