Stathmin regulates mutant p53 stability and transcriptional activity in ovarian cancer
- PMID: 23610071
- PMCID: PMC3662314
- DOI: 10.1002/emmm.201201504
Stathmin regulates mutant p53 stability and transcriptional activity in ovarian cancer
Erratum in
- EMBO Mol Med. 2014 Feb;6(2):295
Abstract
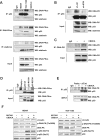
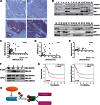
Stathmin is a p53-target gene, frequently overexpressed in late stages of human cancer progression. Type II High Grade Epithelial Ovarian Carcinomas (HG-EOC) represents the only clear exception to this observation. Here, we show that stathmin expression is necessary for the survival of HG-EOC cells carrying a p53 mutant (p53(MUT) ) gene. At molecular level, stathmin favours the binding and the phosphorylation of p53(MUT) by DNA-PKCS , eventually modulating p53(MUT) stability and transcriptional activity. Inhibition of stathmin or DNA-PKCS impaired p53(MUT) -dependent transcription of several M phase regulators, resulting in M phase failure and EOC cell death, both in vitro and in vivo. In primary human EOC a strong correlation exists between stathmin, DNA-PKCS , p53(MUT) overexpression and its transcriptional targets, further strengthening the relevance of the new pathway here described. Overall our data support the hypothesis that the expression of stathmin and p53 could be useful for the identification of high risk patients that will benefit from a therapy specifically acting on mitotic cancer cells.
Copyright © 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures






Similar articles
-
Stathmin is overexpressed and regulated by mutant p53 in oral squamous cell carcinoma.J Exp Clin Cancer Res. 2017 Aug 14;36(1):109. doi: 10.1186/s13046-017-0575-4. J Exp Clin Cancer Res. 2017. PMID: 28806997 Free PMC article.
-
Protumorigenic overexpression of stathmin/Op18 by gain-of-function mutation in p53 in human hepatocarcinogenesis.Hepatology. 2007 Sep;46(3):759-68. doi: 10.1002/hep.21736. Hepatology. 2007. PMID: 17663418
-
Silencing of stathmin induces tumor-suppressor function in breast cancer cell lines harboring mutant p53.Oncogene. 2007 Feb 15;26(7):1003-12. doi: 10.1038/sj.onc.1209864. Epub 2006 Aug 14. Oncogene. 2007. PMID: 16909102
-
The individualization of cancer therapy: the unexpected role of p53.Trans Am Clin Climatol Assoc. 2006;117:85-101; discussion 101. Trans Am Clin Climatol Assoc. 2006. PMID: 18528466 Free PMC article. Review.
-
Stathmin 1 in normal and malignant hematopoiesis.BMB Rep. 2014 Dec;47(12):660-5. doi: 10.5483/bmbrep.2014.47.12.020. BMB Rep. 2014. PMID: 24667172 Free PMC article. Review.
Cited by
-
Overexpression of Stathmin 1 Predicts Poor Prognosis and Promotes Cancer Cell Proliferation and Migration in Ovarian Cancer.Dis Markers. 2022 Feb 9;2022:3554100. doi: 10.1155/2022/3554100. eCollection 2022. Dis Markers. 2022. PMID: 35186166 Free PMC article.
-
Stathmin is overexpressed and regulated by mutant p53 in oral squamous cell carcinoma.J Exp Clin Cancer Res. 2017 Aug 14;36(1):109. doi: 10.1186/s13046-017-0575-4. J Exp Clin Cancer Res. 2017. PMID: 28806997 Free PMC article.
-
5-Methoxyisatin N(4)-Pyrrolidinyl Thiosemicarbazone (MeOIstPyrd) Restores Mutant p53 and Inhibits the Growth of Skin Cancer Cells, In Vitro.ACS Omega. 2023 Aug 24;8(35):31998-32016. doi: 10.1021/acsomega.3c03824. eCollection 2023 Sep 5. ACS Omega. 2023. PMID: 37692215 Free PMC article.
-
KDM4C-mediated senescence defense is a targetable vulnerability in gastric cancer harboring TP53 mutations.Clin Epigenetics. 2023 Oct 17;15(1):163. doi: 10.1186/s13148-023-01579-6. Clin Epigenetics. 2023. PMID: 37848946 Free PMC article.
-
Epigenetic control of autophagy by microRNAs in ovarian cancer.Biomed Res Int. 2014;2014:343542. doi: 10.1155/2014/343542. Epub 2014 Apr 30. Biomed Res Int. 2014. PMID: 24877083 Free PMC article.
References
-
- Anglesio MS, Arnold JM, George J, Tinker AV, Tothill R, Waddell N, Simms L, Locandro B, Fereday S, Traficante N, et al. Mutation of ERBB2 provides a novel alternative mechanism for the ubiquitous activation of RAS-MAPK in ovarian serous low malignant potential tumors. Mol Cancer Res. 2008;6:1678–1690. - PMC - PubMed
-
- Belletti B, Baldassarre G. Stathmin: a protein with many tasks. New biomarker and potential target in cancer. Expert Opin Ther Targets. 2011;15:1249–1266. - PubMed
-
- Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol. 2002;14:18–24. - PubMed
-
- Cassinelli G, Supino R, Perego P, Polizzi D, Lanzi C, Pratesi G, Zunino F. A role for loss of p53 function in sensitivity of ovarian carcinoma cells to taxanes. Int J Cancer. 2001;92:738–747. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

