Role of host cell traversal by the malaria sporozoite during liver infection
- PMID: 23610126
- PMCID: PMC3646492
- DOI: 10.1084/jem.20121130
Role of host cell traversal by the malaria sporozoite during liver infection
Abstract
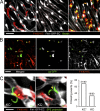
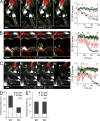
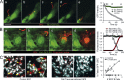
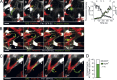
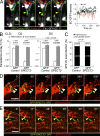
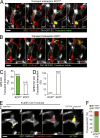
Malaria infection starts when the sporozoite stage of the Plasmodium parasite is injected into the skin by a mosquito. Sporozoites are known to traverse host cells before finally invading a hepatocyte and multiplying into erythrocyte-infecting forms, but how sporozoites reach hepatocytes in the liver and the role of host cell traversal (CT) remain unclear. We report the first quantitative imaging study of sporozoite liver infection in rodents. We show that sporozoites can cross the liver sinusoidal barrier by multiple mechanisms, targeting Kupffer cells (KC) or endothelial cells and associated or not with the parasite CT activity. We also show that the primary role of CT is to inhibit sporozoite clearance by KC during locomotion inside the sinusoid lumen, before crossing the barrier. By being involved in multiple steps of the sporozoite journey from the skin to the final hepatocyte, the parasite proteins mediating host CT emerge as ideal antibody targets for vaccination against the parasite.
Figures

References
-
- Amino R., Giovannini D., Thiberge S., Gueirard P., Boisson B., Dubremetz J.F., Prévost M.C., Ishino T., Yuda M., Ménard R. 2008. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe. 3:88–96 10.1016/j.chom.2007.12.007 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

