Histone chaperone FACT action during transcription through chromatin by RNA polymerase II
- PMID: 23610384
- PMCID: PMC3651417
- DOI: 10.1073/pnas.1222198110
Histone chaperone FACT action during transcription through chromatin by RNA polymerase II
Abstract
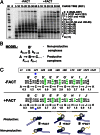
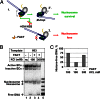
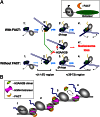
FACT (facilitates chromatin transcription) is a histone chaperone that promotes chromatin recovery during transcription, with additional roles in cell differentiation. Although several models of the action of FACT during transcription have been proposed, they remain to be experimentally evaluated. Here we show that human FACT (hFACT) facilitates transcription through chromatin and promotes nucleosome recovery in vitro. FACT action depends on the presence of histone H2A/H2B dimers in the nucleosome. Kinetic analysis suggests that hFACT decreases the lifetime of nonproductive RNA polymerase II (Pol II)-nucleosome complexes and facilitates the formation of productive complexes containing nucleosomal DNA partially uncoiled from the octamer. Taken together, our data suggest that hFACT interacts with DNA-binding surfaces of H2A/H2B dimers, facilitating uncoiling of DNA from the histone octamer. Thus, hFACT-H2A/H2B interactions play a key role in overcoming the nucleosomal barrier by Pol II and promoting nucleosome survival during transcription.
Keywords: DNA uncoiling; dynamics; elongation; mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Experimental analysis of hFACT action during Pol II transcription in vitro.Methods Mol Biol. 2015;1276:315-26. doi: 10.1007/978-1-4939-2392-2_19. Methods Mol Biol. 2015. PMID: 25665573 Free PMC article.
-
FACT facilitates transcription-dependent nucleosome alteration.Science. 2003 Aug 22;301(5636):1090-3. doi: 10.1126/science.1085703. Science. 2003. PMID: 12934006
-
N-Terminal Tails of Histones H2A and H2B Differentially Affect Transcription by RNA Polymerase II In Vitro.Cells. 2022 Aug 10;11(16):2475. doi: 10.3390/cells11162475. Cells. 2022. PMID: 36010552 Free PMC article.
-
[Structure and function of histone chaperone FACT].Mol Biol (Mosk). 2015 Nov-Dec;49(6):891-904. doi: 10.7868/S0026898415060026. Mol Biol (Mosk). 2015. PMID: 26710768 Review. Russian.
-
Mechanism of transcription through a nucleosome by RNA polymerase II.Biochim Biophys Acta. 2013 Jan;1829(1):76-83. doi: 10.1016/j.bbagrm.2012.08.015. Epub 2012 Sep 6. Biochim Biophys Acta. 2013. PMID: 22982194 Free PMC article. Review.
Cited by
-
Nucleosomal Barrier to Transcription: Structural Determinants and Changes in Chromatin Structure.Biochem Mol Biol J. 2016;2(2):8. doi: 10.21767/2471-8084.100017. Epub 2016 May 30. Biochem Mol Biol J. 2016. PMID: 27754494 Free PMC article.
-
A systematic mutational analysis of a histone H3 residue in budding yeast provides insights into chromatin dynamics.G3 (Bethesda). 2015 Feb 23;5(5):741-9. doi: 10.1534/g3.115.017376. G3 (Bethesda). 2015. PMID: 25711831 Free PMC article.
-
Human FACT subunits coordinate to catalyze both disassembly and reassembly of nucleosomes.Cell Rep. 2022 Dec 27;41(13):111858. doi: 10.1016/j.celrep.2022.111858. Cell Rep. 2022. PMID: 36577379 Free PMC article.
-
Organization and regulation of gene transcription.Nature. 2019 Sep;573(7772):45-54. doi: 10.1038/s41586-019-1517-4. Epub 2019 Aug 28. Nature. 2019. PMID: 31462772 Review.
-
Structural basis of nucleosome retention during transcription elongation.Science. 2022 Jun 17;376(6599):1313-1316. doi: 10.1126/science.abo3851. Epub 2022 Jun 16. Science. 2022. PMID: 35709268 Free PMC article.
References
-
- Orphanides G, LeRoy G, Chang CH, Luse DS, Reinberg D. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell. 1998;92(1):105–116. - PubMed
-
- Garcia-Ramirez M, Dong F, Ausio J. Role of the histone “tails” in the folding of oligonucleosomes depleted of histone H1. J Biol Chem. 1992;267(27):19587–19595. - PubMed
-
- Orphanides G, Wu WH, Lane WS, Hampsey M, Reinberg D. The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature. 1999;400(6741):284–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM58650/GM/NIGMS NIH HHS/United States
- R01 GM055310/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 GM058650/GM/NIGMS NIH HHS/United States
- R01 GM064844/GM/NIGMS NIH HHS/United States
- R37GM037120/GM/NIGMS NIH HHS/United States
- R37 GM037120/GM/NIGMS NIH HHS/United States
- R01 GM051966/GM/NIGMS NIH HHS/United States
- R37 GM051966/GM/NIGMS NIH HHS/United States
- P01 GM088409/GM/NIGMS NIH HHS/United States
- GM064844/GM/NIGMS NIH HHS/United States
- GM51966/GM/NIGMS NIH HHS/United States
- GM55310/GM/NIGMS NIH HHS/United States
- GM088409/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

