Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production
- PMID: 23610393
- PMCID: PMC3651434
- DOI: 10.1073/pnas.1218599110
Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production
Abstract
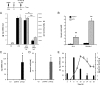
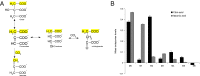
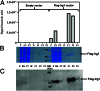
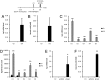
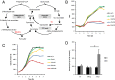
Immunoresponsive gene 1 (Irg1) is highly expressed in mammalian macrophages during inflammation, but its biological function has not yet been elucidated. Here, we identify Irg1 as the gene coding for an enzyme producing itaconic acid (also known as methylenesuccinic acid) through the decarboxylation of cis-aconitate, a tricarboxylic acid cycle intermediate. Using a gain-and-loss-of-function approach in both mouse and human immune cells, we found Irg1 expression levels correlating with the amounts of itaconic acid, a metabolite previously proposed to have an antimicrobial effect. We purified IRG1 protein and identified its cis-aconitate decarboxylating activity in an enzymatic assay. Itaconic acid is an organic compound that inhibits isocitrate lyase, the key enzyme of the glyoxylate shunt, a pathway essential for bacterial growth under specific conditions. Here we show that itaconic acid inhibits the growth of bacteria expressing isocitrate lyase, such as Salmonella enterica and Mycobacterium tuberculosis. Furthermore, Irg1 gene silencing in macrophages resulted in significantly decreased intracellular itaconic acid levels as well as significantly reduced antimicrobial activity during bacterial infections. Taken together, our results demonstrate that IRG1 links cellular metabolism with immune defense by catalyzing itaconic acid production.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
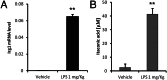
References
-
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12(7):388–399. - PubMed
-
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. - PubMed
-
- Mantovani A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. - PubMed
-
- Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009;210(1-2):3–12. - PubMed
-
- Tangsudjai S, et al. Involvement of the MyD88-independent pathway in controlling the intracellular fate of Burkholderia pseudomallei infection in the mouse macrophage cell line RAW 264.7. Microbiol Immunol. 2010;54(5):282–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

