Renal intercalated cells are rather energized by a proton than a sodium pump
- PMID: 23610411
- PMCID: PMC3651478
- DOI: 10.1073/pnas.1221496110
Renal intercalated cells are rather energized by a proton than a sodium pump
Abstract
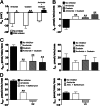
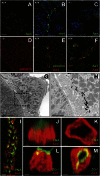
The Na(+) concentration of the intracellular milieu is very low compared with the extracellular medium. Transport of Na(+) along this gradient is used to fuel secondary transport of many solutes, and thus plays a major role for most cell functions including the control of cell volume and resting membrane potential. Because of a continuous leak, Na(+) has to be permanently removed from the intracellular milieu, a process that is thought to be exclusively mediated by the Na(+)/K(+)-ATPase in animal cells. Here, we show that intercalated cells of the mouse kidney are an exception to this general rule. By an approach combining two-photon imaging of isolated renal tubules, physiological studies, and genetically engineered animals, we demonstrate that inhibition of the H(+) vacuolar-type ATPase (V-ATPase) caused drastic cell swelling and depolarization, and also inhibited the NaCl absorption pathway that we recently discovered in intercalated cells. In contrast, pharmacological blockade of the Na(+)/K(+)-ATPase had no effects. Basolateral NaCl exit from β-intercalated cells was independent of the Na(+)/K(+)-ATPase but critically relied on the presence of the basolateral ion transporter anion exchanger 4. We conclude that not all animal cells critically rely on the sodium pump as the unique bioenergizer, but can be replaced by the H(+) V-ATPase in renal intercalated cells. This concept is likely to apply to other animal cell types characterized by plasma membrane expression of the H(+) V-ATPase.
Keywords: ion transporter; plasma membrane; proton pump.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Edelman IS. Thyroid thermogenesis. N Engl J Med. 1974;290(23):1303–1308. - PubMed
-
- Morth JP, et al. A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nat Rev Mol Cell Biol. 2011;12(1):60–70. - PubMed
-
- Brown D, Breton S. Mitochondria-rich, proton-secreting epithelial cells. J Exp Biol. 1996;199(Pt 11):2345–2358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

