Transient formation of water-conducting states in membrane transporters
- PMID: 23610412
- PMCID: PMC3651479
- DOI: 10.1073/pnas.1218986110
Transient formation of water-conducting states in membrane transporters
Abstract
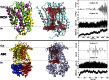
Membrane transporters rely on highly coordinated structural transitions between major conformational states for their function, to prevent simultaneous access of the substrate binding site to both sides of the membrane--a mode of operation known as the alternating access model. Although this mechanism successfully accounts for the efficient exchange of the primary substrate across the membrane, accruing evidence on significant water transport and even uncoupled ion transport mediated by transporters has challenged the concept of perfect mechanical coupling and coordination of the gating mechanism in transporters, which might be expected from the alternating access model. Here, we present a large set of extended equilibrium molecular dynamics simulations performed on several classes of membrane transporters in different conformational states, to test the presence of the phenomenon in diverse transporter classes and to investigate the underlying molecular mechanism of water transport through membrane transporters. The simulations reveal spontaneous formation of transient water-conducting (channel-like) states allowing passive water diffusion through the lumen of the transporters. These channel-like states are permeable to water but occluded to substrate, thereby not hindering the uphill transport of the primary substrate, i.e., the alternating access model remains applicable to the substrate. The rise of such water-conducting states during the large-scale structural transitions of the transporter protein is indicative of imperfections in the coordinated closing and opening motions of the cytoplasmic and extracellular gates. We propose that the observed water-conducting states likely represent a universal phenomenon in membrane transporters, which is consistent with their reliance on large-scale motion for function.
Keywords: ABC transporters; LeuT-fold transporters; major facilitator superfamily; neurotransmitter transporters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Locking Two Rigid-body Bundles in an Outward-Facing Conformation: The Ion-coupling Mechanism in a LeuT-fold Transporter.Sci Rep. 2019 Dec 20;9(1):19479. doi: 10.1038/s41598-019-55722-6. Sci Rep. 2019. PMID: 31862903 Free PMC article.
-
Structural elements required for coupling ion and substrate transport in the neurotransmitter transporter homolog LeuT.Proc Natl Acad Sci U S A. 2018 Sep 18;115(38):E8854-E8862. doi: 10.1073/pnas.1716870115. Epub 2018 Sep 4. Proc Natl Acad Sci U S A. 2018. PMID: 30181291 Free PMC article.
-
Microscopic Characterization of Membrane Transporter Function by In Silico Modeling and Simulation.Methods Enzymol. 2016;578:373-428. doi: 10.1016/bs.mie.2016.05.042. Epub 2016 Jul 11. Methods Enzymol. 2016. PMID: 27497175 Free PMC article. Review.
-
Identification of a second substrate-binding site in solute-sodium symporters.J Biol Chem. 2015 Jan 2;290(1):127-41. doi: 10.1074/jbc.M114.584383. Epub 2014 Nov 14. J Biol Chem. 2015. PMID: 25398883 Free PMC article.
-
Mechanistic aspects of sodium-binding sites in LeuT-like fold symporters.Biol Chem. 2013 May;394(5):641-8. doi: 10.1515/hsz-2012-0336. Biol Chem. 2013. PMID: 23362203 Review.
Cited by
-
Conformational Changes in Two Inter-Helical Loops of Mhp1 Membrane Transporter.PLoS One. 2015 Jul 17;10(7):e0133388. doi: 10.1371/journal.pone.0133388. eCollection 2015. PLoS One. 2015. PMID: 26186341 Free PMC article.
-
A critique of the alternating access transporter model of uniport glucose transport.Biophys Rep. 2018;4(6):287-299. doi: 10.1007/s41048-018-0076-9. Epub 2018 Nov 16. Biophys Rep. 2018. PMID: 30596138 Free PMC article. No abstract available.
-
Disulfide cross-linking of transport and trimerization domains of a neuronal glutamate transporter restricts the role of the substrate to the gating of the anion conductance.J Biol Chem. 2014 Apr 18;289(16):11175-11182. doi: 10.1074/jbc.M114.550277. Epub 2014 Feb 28. J Biol Chem. 2014. PMID: 24584931 Free PMC article.
-
Structural dynamics of the monoamine transporter homolog LeuT from accelerated conformational sampling and channel analysis.Proteins. 2014 Oct;82(10):2289-302. doi: 10.1002/prot.24588. Epub 2014 May 9. Proteins. 2014. PMID: 24753369 Free PMC article.
-
Mouse Slc4a11 expressed in Xenopus oocytes is an ideally selective H+/OH- conductance pathway that is stimulated by rises in intracellular and extracellular pH.Am J Physiol Cell Physiol. 2016 Dec 1;311(6):C945-C959. doi: 10.1152/ajpcell.00259.2016. Epub 2016 Sep 28. Am J Physiol Cell Physiol. 2016. PMID: 27681179 Free PMC article.
References
-
- DeFelice LJ. Transporter structure and mechanism. Trends Neurosci. 2004;27(6):352–359. - PubMed
-
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211(5052):969–970. - PubMed
-
- Forrest LR, Krämer R, Ziegler C. The structural basis of secondary active transport mechanisms. Biochim Biophys Acta. 2011;1807(2):167–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

